Meloxicam is a non-steroidal anti-inflammatory drug with analgesic and antipyretic effects. It is used as injections (deep intramuscular), tablets (orally) and suppositories (rectally). The medicine is affordable and gives a quick effect. Used only in consultation with a doctor, dispensed from pharmacies with a prescription.
"Meloxicam" is a drug with the active ingredient of the same name. It is a non-steroidal anti-inflammatory drug. It has antipyretic, analgesic and analgesic effects.
Available in 3 dosage forms:
- solution in ampoules;
- pills;
- suppositories (candles).
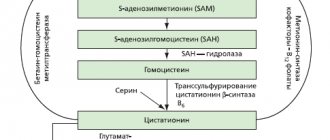
The active component of Meloxicam is a derivative of organic enolic acid. It inhibits the activity of the enzyme cyclooxygenase-2 and slows down the formation of prostaglandins. As a result, inflammatory processes are stopped, the lesion is reduced, resulting in an analgesic effect.
The drug is released only with a prescription. It can be stored at room temperature (no more than 25 degrees) out of the reach of children. The shelf life is 2 years from the date of production.
Meloxicam: release forms and application features
The drug Meloxicam belongs to the group of non-steroidal anti-inflammatory drugs (NSAIDs). Its main effects:
- anti-inflammatory;
- antipyretic;
- anesthetic.
Due to the pronounced inhibition of the inflammatory process at all stages of its development, Meloxicam is used for the treatment of rheumatological diseases.
The main mechanism of action is inhibition of the production of prostaglandins (substances involved in the development of inflammation).
Why is Meloxicam better?
Meloxicam belongs to the group of selective NSAIDs. It has a lesser effect on the production of cyclooxygenase-1, an enzyme that regulates many metabolic processes in the body, in particular the production of mucus in the stomach and gastric juice. COX-1 has gastroprotective properties, therefore, when using non-selective NSAIDs, complications often arise in the form of erosions, ulcers and bleeding in the gastrointestinal tract.
COX-2 is a pro-inflammatory enzyme that regulates the production of prostaglandins. It increases only in the presence of inflammation in the body and regulates its course.
Due to the selectivity of action only on COX-2 and to a lesser extent on COX-1, Meloxicam has better tolerability compared to analogues. With its use, complications from the digestive tract occur less frequently.
Can it be taken by children and pregnant women?
The drug is contraindicated for children under 16 years of age.
Pregnant women and those planning a pregnancy should use Meloxicam only when absolutely necessary and in the lowest possible doses.
Since the drug Meloxicam reduces the formation of prostaglandins, this can negatively affect a woman's reproductive rate. In the first trimester, use of the drug may increase the risk of premature abortion and fetal malformations. In the second and third trimesters, taking Meloxicam can have a toxic effect on the cardiopulmonary system and fetal kidneys.
In addition, during treatment, bleeding time may increase due to the antiplatelet effect of the drug and the duration of labor may increase due to the effect on uterine contractions.
Contraindications and adverse events
The drug is not prescribed for:
- hypersensitivity to its components;
- severe heart failure;
- in the third trimester of pregnancy;
- up to 16 years old;
- a history of bleeding from the gastrointestinal tract;
- active ulcers or erosions in the digestive tract;
- severe kidney failure;
- simultaneous use with anticoagulants and blood clotting disorders;
- therapy of postoperative pain during CABG (coronary artery bypass grafting).
The most common side effects:
- the occurrence of ulcers, erosions in the digestive tract and an increased risk of bleeding, especially in elderly patients;
- increased risk of cardiovascular events (heart attack, stroke);
- swelling;
- increase in blood pressure (blood pressure);
- heart failure;
- other intestinal manifestations: ulcerative stomatitis, vomiting, bloating, nausea, blood in the stool, abdominal pain, loose stools or constipation, vomiting with blood, relapse of Crohn's disease, rarely gastritis.
The instructions contain the entire list of possible adverse reactions - be sure to read them before use.
You can find out the current price for Meloxicam and buy it on the Pharmacy 911 website.
Drug interactions
Combined use with myelotoxic drugs increases the side effects of Meloxicam on the blood. If you take the drug together with non-steroidal anti-inflammatory medications, the risk of bleeding in the stomach and intestines, as well as exacerbation of ulcers, increases.
If you take Cholestyramine, the drug promotes the rapid elimination of Meloxicam. When using Heparin, Ticlopidine, drugs to lower blood pressure, and indirect anticoagulants, the risk of bleeding increases. When taken together with Cyclosporine, the likelihood of kidney-related side effects increases.
Popular questions about Meloxicam
What is Meloxicam for?
It is used to reduce pain and reduce the severity of inflammation in such rheumatological diseases as: osteoarthritis, rheumatoid arthritis, ankylosing spondylitis.
How to give Meloxicam injections?
The contents of the ampoule are injected intramuscularly, deep into the upper outer quadrant of the gluteal muscle.
During the injection, it is important to treat the injection site with an antiseptic solution. It is recommended to alternate injection sites. Before injecting the medicine into the muscle, you need to make sure that the syringe needle does not fall into the vessel. If you experience severe pain during the administration of the drug, the injection should be interrupted.
Dosage - 1 ampoule (15 mg) per day. It is important not to exceed the daily dose. The course of treatment is determined by the doctor, but, as a rule, the drug is used in the form of injections for no more than 2-3 days, and then switched to tablet form.
How to take Meloxicam tablets?
Take 7.5 or 15 mg once every 24 hours.
The daily dosage should not exceed 15 mg. Take the tablet with meals. For elderly patients and patients with renal failure, the daily dose should be reduced to 7.5 mg.
How long can you take Meloxicam?
The duration of therapy is determined by the doctor, but the shorter it is, the lower the risk of developing health complications from the gastrointestinal tract and cardiovascular system.
Meloxicam in the treatment of chronic diseases of the musculoskeletal system
Millions of people suffer from pain in the joints and spine due to various rheumatic diseases; pain in the periarticular tissues and muscles accompanies many diseases or occurs as an independent suffering. The most common diseases associated with chronic pain syndrome are rheumatoid arthritis (RA), spondyloarthropathy (SA) and osteoarthritis (OA). At various periods of life, pain due to damage to components of the musculoskeletal system occurs in 20–45% of the world's population, more often in women than men, and in older age groups [1].
The chronic nature of the pain syndrome of most rheumatic diseases is due to the development of inflammation in the synovium of the joints due to the overproduction of a large number of proinflammatory agents, modulation of the function of immunocompetent cells and their proliferation, and the destructive action of proteases [2]. Even OA, a disease that is usually classified as degenerative joint disease, is also characterized by the development of synovitis and is an indication for the prescription of drugs that stop the inflammatory process. Pain syndrome inevitably accompanies inflammation in the joint, although its intensity does not always correlate with the severity of inflammation.
Anti-inflammatory therapy for chronic inflammatory arthropathies is carried out for a long time, since in diseases such as RA, SA, spontaneous remissions are practically absent. Therefore, patients are forced to take medications that reduce pain and inflammation for many months and years. The need for long-term therapy imposes requirements for symptomatic treatment on the rapid development of the effect, the severity of the effect, and tolerability during long-term use.
Treatment of patients with OA is complicated by the elderly age of most patients, the presence of concomitant diseases and the need for concomitant therapy. All of these factors are risk factors for the development of complications of anti-inflammatory drugs. The problem of comorbidity in OA always worries clinicians. In recent years, new data have been emerging on the frequency of concomitant pathologies in patients with diseases of the musculoskeletal system, primarily in patients with OA. According to a survey of more than 9000 patients in Serbia [3] (Fig. 1), concomitant pathology was detected in more than 60% of patients.
In a case-control study [4] conducted in the UK, when comparing the frequency of comorbidity in 11,375 patients with OA compared with 11,780 persons without OA, an increase in the frequency of OA was found: obesity by 2.25 times, gastritis by 1.98 times, phlebitis by 1.8 times, diaphragmatic hernia by 1.8 times, coronary heart disease (CHD) by 1.73 times, intestinal diverticulosis by 1.63 times. According to many authors, the most common comorbid conditions are arterial hypertension, coronary heart disease and diabetes [3–9]. On the one hand, they are well-known risk factors for intolerance to nonsteroidal anti-inflammatory drugs (NSAIDs). On the other hand, taking NSAIDs aggravates the course of arterial hypertension (AH), reduces the effectiveness of antihypertensive therapy, and can aggravate congestive heart failure (CHF) [10–13]. The increase in the frequency of NSAID-gastropathy in the elderly is well known (Fig. 2). Less covered in the literature is that taking NSAIDs doubles the risk of developing CHF and doubles the risk of hospitalization for CHF [12, 13], and in people with CHF, taking NSAIDs increases the risk of its increase by 10.5 times [ 12].
Thus, the requirements for drug therapy that can reduce the severity of inflammation and pain, taking into account the need for long-term use of drugs, are determined by the severity of the analgesic and anti-inflammatory effect and their safety.
Meloxicam, a selective cyclooxygenase-2 (COX-2) inhibitor, is widely used in medical practice. Meloxicam is a derivative of hydroxycamic acid and has a long half-life: the maximum concentration (Cmax) in plasma after taking 15 mg of the drug is reached after 7 hours, the half-life is 20–24 hours, so it is prescribed once a day at a dose of 7.5 or 15 mg, which is convenient for the patient. Meloxicam is structurally different from other COX-2 inhibitors, such as the coxibs, and binds to the top of the COX-2 channel rather than to the side of the enzyme like celecoxib. Meloxicam binds well to plasma proteins (99.5%) and easily penetrates into the synovial fluid, where its concentration is 45–57% of the plasma concentration [10].
The effectiveness of meloxicam was assessed in a number of large randomized clinical trials (RCTs) in OA (MELISSA studies, n = 9323; SELECT, n = 8656; in the USA, n = 774), RA (n = 894), ankylosing spondylitis (AS) ( n = 473) (Fig. 2, 3).
It has been shown that the effectiveness of meloxicam in the treatment of patients with OA is equal to the effectiveness of non-selective NSAIDs (diclofenac, piroxicam) [14], and the tolerability is much better [17, 18] (Fig. 4). The drug demonstrated equivalent effectiveness with non-selective NSAIDs in both RA and ankylosing spondylitis [15, 16].
Recently, a new parenteral form of meloxicam for intramuscular administration has been introduced into medical practice. The need to create this form is due to the fact that, due to the significant half-life of meloxicam, its concentration when taking the tablet form stabilizes in the patient’s blood only on days 3–4. Therefore, the parenteral form was developed to quickly relieve severe or acute pain. Pharmacokinetic studies have shown that intramuscular use of meloxicam leads to faster absorption of the drug than when administered orally; maximum plasma concentration is achieved within 1.5 hours after IM administration compared to 5–7 hours after oral administration [19]. In this case, 90% of Cmax is achieved within 30–50 minutes after injection. This increase in absorption determines the faster onset of action of meloxicam administered IM compared to oral administration (Fig. 5).
In order for IM administration to be considered as an alternative to the oral route of administration, very good local tolerability is required. However, many NSAIDs are poorly tolerated when administered intramuscularly, causing local tissue irritation and necrosis, often in combination with systemic adverse events [20]. When working on rabbits, it was shown that the local tolerability of meloxicam is better than that of other NSAIDs. After its intramuscular administration, no histopathological changes were detected, while when using piroxicam or diclofenac, a large area of necrosis developed.
The advantage of using the IM form of meloxicam compared to the tablet form has been demonstrated in RA [20, 21], OA [21], and lumbar ischialgia syndrome [22]. A Russian multicenter study examining the effectiveness of the intramuscular form of meloxicam in the treatment of 670 patients with joint pathology (OA - 384 patients and RA - 286 patients) showed that the effect when meloxicam is administered into the muscle develops in most patients within the first hour after 1- injection, increases during the first three days and then continues to increase when switching to the oral form, so that by the end of the course of treatment, a significant decrease in pain (at rest and with movement) and improvement in function were obtained (Fig. 6).
This step-by-step method of prescribing meloxicam - intramuscular injection of the drug for three days and subsequent switching to tablet form can be especially useful in the treatment of arthrosis of the intervertebral joints and other causes of back pain (osteochondrosis), where the severity and severity of pain can be much greater than in OA of peripheral joints. When comparing the effectiveness and tolerability of parenteral forms of meloxicam and piroxicam in the treatment of acute pain in the shoulder joint in 599 patients, after 7 days the degree of pain reduction was equal in both groups of patients. However, meloxicam had a faster onset of action: in the first 3 days of therapy, pain decreased with injections of 7.5–15 mg of meloxicam in more patients than among those receiving injections of 20 mg of piroxicam [23].
This is explained by differences in the pharmacokinetics of these two drugs (meloxicam reaches stable plasma concentrations after 3–4 days, and piroxicam after 7–10 days). It should be borne in mind that piroxicam is one of the most poorly tolerated NSAIDs, the use of which in old age in patients with OA is highly undesirable, therefore piroxicam is usually used in the treatment of young people who do not have concomitant pathologies. A rapid reduction in spontaneous pain and pain during movement occurs when using the parenteral form of meloxicam in the treatment of exacerbation of lumbar ischialgic syndrome (radiculopathy, myofascial and muscletonic disorders) [22], when the speed of development of the effect is necessary not only because of the severity of pain, but also to prevent the development of the effect “secondary hyperalgesia” and chronicity of the pathological process. The administration of meloxicam intramuscularly made it possible to achieve a reduction in spontaneous pain by approximately 2 times 1 hour after the 1st injection, and pain during movement by more than 2 times, and after the 3rd injection by 77% and 78%, respectively.
The safety of meloxicam in the treatment of major rheumatological diseases of the joints and spine was assessed in a 1999 meta-analysis [24], which included data from 10 studies (Fig. 7).
According to a meta-analysis of the results of 10 published studies, meloxicam had advantages over diclofenac, piroxicam and naproxen:
- in terms of the number of complications from the gastrointestinal tract: risk reduction by ~36%;
- in terms of the frequency of withdrawal due to complications from the gastrointestinal tract: risk reduction by ~41%;
- in terms of the frequency of perforations, ulcers, bleeding from the upper gastrointestinal tract: risk reduction by ~ 48%;
- in terms of the incidence of dyspepsia: risk reduction by ~27%.
Randomized clinical trials have shown the high safety of meloxicam. Over the past decade, extensive experience has been gained in the use of meloxicam in real clinical practice, when treatment is carried out on patients of all ages, with concomitant and sometimes severe diseases, and receiving various medications, which complicates the implementation of analgesic and anti-inflammatory therapy.
Presented by Zeidler H. et al. [25] data on the results of treatment of 13,307 patients with joint pathology in routine medical practice in Germany make it possible to assess the place of meloxicam in the opinion of 2,155 doctors. The majority of patients (60%) received other NSAIDs before prescribing meloxicam: in 43.2% of cases their prescription was not effective, and in every 5th patient it was intolerable. In this group of patients, the frequency of adverse reactions leading to drug discontinuation when taking meloxicam 7.5 mg/day was 0.7% and 15 mg/day - 0.6%; the development of complicated gastrointestinal ulcers was noted in 2 out of 8652 patients receiving meloxicam 7.5 mg/day, and in another 2 out of 4448 patients with a daily dose of meloxicam 15 mg. Let us recall that the use of non-selective NSAIDs leads to the development of ulcers of the upper gastrointestinal tract in 12–19% of cases, and complicated ulcers in approximately 0.4% of cases [26]. Moreover, in many patients (up to 80%), the occurrence of ulcerative damage to the gastrointestinal mucosa occurs painlessly [26], which prevents the timely administration of gastroprotective therapy.
In a pharmacoeconomic study of the safety of meloxicam in patients with risk factors for the development of NSAID gastropathy, compared with other NSAIDs [27], obvious evidence was obtained of significantly better tolerability of meloxicam (Table 1),
including the frequency of bleeding from the upper gastrointestinal tract (Fig. 8).
The problems of NSAID gastropathy are quite often discussed in the literature. Data on kidney complications are less frequently reported. Non-selective NSAIDs cause a decrease in the level of renal prostaglandins, which leads to impaired Na excretion, fluid retention, development of arterial hypertension or worsening of its course. At the same time, the effectiveness of antihypertensive drugs decreases, which dictates the need for careful monitoring of blood pressure and dose adjustment of beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, and diuretics. According to the results of pathological and anatomical studies, interstitial nephritis is observed in 60–100% of patients with RA who are forced to take full therapeutic doses of non-selective NSAIDs for years.
The development of acute renal failure (ARF) is possible. A thorough analysis of the risk of developing acute renal failure in elderly patients was carried out in the USA [28]. According to the program for assessing the effectiveness and safety of drugs prescribed between 1999 and 2004. in persons over 65 years of age, the side effects of NSAIDs were assessed when taken for 6 months or more. Patients receiving two NSAIDs simultaneously were excluded from the study. Of 183,446 patients with a mean age of 78 years, AKI leading to hospitalization occurred in 870 patients. The most common NSAID prescribed to this group of patients was celecoxib, which was taken by every third patient. In table Table 2 shows the relative risk and 95% confidence interval of the development of acute renal failure when taking various NSAIDs in comparison with celecoxib.
A significant increase in the risk of developing acute renal failure by 50% and 100% was obtained for ibuprofen and indomethacin, respectively. The table shows that meloxicam has the lowest risk of developing acute renal failure among the selective and non-selective NSAIDs analyzed.
Both of these summaries are of undoubted interest to clinicians, since they are based not on the results of scientific research, but on the basis of reports from practicing physicians.
In recent years, another aspect of the safety of NSAIDs has been discussed - the possibility of aggravating the course of cardiovascular diseases. Theoretically, the increase in thrombogenic risk of selective cyclooxygenase type 2 inhibitors may be due to their inhibition of prostaglandin-I2 (“antithrombogenic” prostaglandin), a relative increase in the synthesis of thromboxane A2 (“thrombogenic” prostaglandin) when used in patients with RA or systemic lupus erythematosus, that is for diseases in which the risk of thrombosis is increased. Following evidence of a slight increase in the risk of cardiovascular disease with rofecoxib [29], a number of additional studies have been conducted on the effects of NSAIDs on cardiovascular function.
In 2001, the results of a meta-analysis of the results of treatment with meloxicam in 27,000 patients were reported [30]. According to these data, the incidence of cardiovascular adverse reactions during treatment with meloxicam was not higher than when using non-selective NSAIDs. There is a report of a trial study of the use of meloxicam 15 mg intravenously for acute coronary syndrome in 60 patients receiving standard treatment with Aspirin and Heparin, compared with 60 patients receiving the same doses of Aspirin and Heparin without meloxicam. It turned out that the addition of meloxicam to standard therapy when assessing the outcome of acute coronary syndrome (angina recurrence, myocardial infarction (MI) or death) led to a decrease in the frequency of negative outcomes from 38.3% to 15% during hospital stay and from 48. 3% to 26.7% 90 days after treatment [31].
The risk of developing acute myocardial infarction with selective and non-selective NSAIDs was assessed in three populations in three countries (UK, Canada and USA) [32]. The aim of the study was to evaluate the risk of MI in patients taking COX-2 inhibitors, meloxicam and other NSAIDs compared with diclofenac; check the comparability of data for three populations; A total of 60,473 NSAID-treated cases and 248,768 control cases were analyzed. The following results were obtained:
- Meloxicam was associated with a reduced risk of MI in one cohort, but no effect was found in two cohorts (although the relative risk was reduced in both studies).
- In one cohort, naproxen was associated with a reduced risk of MI, and in two, the risk was slightly increased.
- In two cohorts, the risk of MI was slightly increased with rofecoxib compared with diclofenac.
- In one cohort, a small increase in risk was found for ibuprofen compared with diclofenac.
- No differences were found between celecoxib and diclofenac.
- In the GPRD (UK), no significant differences were found, although the relative risk for meloxicam was below 1.0 and for naproxen it was slightly increased.
- The RAMQ (Canada) found a small statistically significant increase in risk with rofecoxib and a decrease in risk with meloxicam and naproxen.
- The US VA found a small but statistically significant increase in risk for rofecoxib, naproxen and ibuprofen and a decrease in risk for meloxicam.
The risk of developing MI in patients with current use of prescription NSAIDs (meloxicam, celecoxib and rofecoxib) compared with current use of diclofenac is presented in
.
Thus, according to the results of RCTs and post-registration studies of meloxicam:
- Its distinct analgesic and anti-inflammatory activity has been revealed in chronic diseases of the joints and spine, as well as in acute pain syndromes (lumbar ischialgia).
- High gastrointestinal tolerability, previously identified in double-blind controlled studies, was confirmed based on the results of real clinical practice in the treatment of many thousands of patient cohorts.
- Large-scale pharmacoepidemiological studies confirm the low risk of severe gastrointestinal side effects previously established in controlled clinical trials and meta-analysis.
- There was no increase in the incidence of cardiovascular toxicity.
These data are confirmed by the IMPROVE study [33], which assessed the “therapeutic success” of meloxicam in OA. The endpoints used to assess “therapeutic success” were: completion of the study without switching to another NSAID, or completion of the study and no need to take an NSAID. Meloxicam was prescribed to 662 patients and other NSAIDs to 647 patients. The end point was reached by 67% of the group of patients taking meloxicam and 45% of patients from the comparison group (p < 0.0005) (Fig. 9).
Treatment cancellation due to adverse reactions occurred in 12% and 20% of patients, respectively, and due to lack of effect - in 16% and 28% of patients. Patients' adherence to taking meloxicam indicates the high effectiveness of this drug.
Literature
- Nasonov E. L. Pain syndrome in pathology of the musculoskeletal system // Doctor. No. 4. 2002. pp. 15–19.
- Scott D. Text book of rheumatology. Philadelphia. 1999.
- Damjanov M. // VI International Meeting, Crete, 2008.
- Kadam UT, Jordan K., Craft PR Clinical comorbidity in patients with osteoarthritis: a case-control study of general practice consultants in England and Walls // Ann. Rheum. Dis. 2004, 63: 408–414.
- Caporali R., Cimmino MA, Sazzi-Puttini P. et. al. Osteoarthritis in general and specialist practice in Italy: the AMICA study // Sem. Arthr. Rheum. 35: 31–37.
- Vertkin A.L., Naumov A.V. Osteoarthrosis: strategy for managing patients with somatic pathology // Breast Cancer. 2007, vol. 15, no. 4.
- Vertkin A.L., Alekseeva L.I., Naumov A.V. et al. Osteoarthrosis in the practice of a general practitioner // RMZh. 2008, volume 16, no. 7.
- Rosemann T., Laux G., Szecsenyi J. Osteoarthritis: quality of life, comorbidities, medication and health service utilization assessed in a large sample of primary care patients // J. Orthoped. Surg. 2007, 2: 12.
- Van Dijk GM, Venhof C., Schellevis F. et al. Comorbidity, limitation in activities and pain in patients with osteoarthritis of the hip or knee // BMS Musculoskeletal Disord. 2008, 9: 95–99.
- Warksman JC Nonselective nonsteroidal anti-inflammatory drugs and cardiovascular risk: are they safe- // Ann. Rharmacother. 2007, 41: 1163–1173.
- Savenkov M.P., Brodskaya S.A., Ivanov S.N., Sudakova N.I. Effect of non-steroidal anti-inflammatory drugs on the antihypertensive effect of ACE inhibitors // Breast Cancer. 203, No. 19: 1056–1059.
- Page J., Henry D. Consumption of NSAIDs and the development of congestive heart failure in elderly patients // Arch. Int. Med. 2000, 160: 777–784.
- Heerdink ER, Leufkens HG, Herings RMC et al. NSAIDs associated with increased risk of congestive heart failure in elderly patients taking diuretics // Arch. Int. Med. 1998, 158: 1108–1112.
- Yocum D., Fleischmann R., Dalgin P. et al. Safety and efficacy of meloxicam in the treatment of osteoarthritis: a 12-week, double-blind, multiple doses, placebo-controlled trial. The Meloxicam Osteoarthritis Investigators // Arch. Int. Med. 2000. V. 160. P. 2947–2954.
- Lemmel EM Efficacy of meloxicam in the treatment of rheumatoid arthritis: a 12-week, double-blind, placebo-controlled trial // J. Rheum. 1997, 24: 282–290.
- Dougados M., Gueguen A., Nakache J.-P. et al. Ankylosing spondilitis: what is the optimal duration of a clinical study- A one-year versus 6-weeks nonsteroidal antiinflammatory drug trial // Rheumatology. 1999. V. 38. P. 235–244.
- Hawkey C., Kahan A., Steinbruck K. et al. Gastrointestinal tolerance of meloxicam compared to diclofenac in osteoarthritis patients. International MELISSA Study Group. Meloxicam Large-scale International Study // J. Rheum. 1998, 37: 937–945.
- Dekueker J., Hawkey C., Kahan A. et al. Improvement in gastrointestinal tolerance of the selective cyclooxigenase (COX)-2 inhibitor meloxicam compared with piroxicam: results of the Safety Large-scale Evaluation of COX-inhibiting Therapies (SELECT) trial in osteoarthritis // J. Rheum. 1998, 37: 946–951.
- Davies NM, Skjodt NM Clinical pharmacokinetics of meloxicam: a cyclo-oxygenase-2 preferential nonsteroidal anti-inflammatory drug // Clin. Pharmacokinet. 1999, V. 36: 115–126.
- Combe B., Velicitat P., Garson N., Bluhmki E. Comparison of intramuscular and oral meloxicam in rheumatoid arthritis patients // Inflamm. Res. 2001, V. 50, Suppl. 1:S10–16.
- Tsvetkova E. S. Efficacy and tolerability of stepwise therapy with movalis (meloxicam) for rheumatic diseases // Ter. archive. 2004, no. 12, 78–80.
- Alekseev V.V. Use of meloxicam in the treatment of lumbar ischialgia syndrome // Breast Cancer. 2003, vol. 7, no. 11: 416–418.
- Vidal L., Kneer W., Baturone M. et al. Meloxicam in acute episodes of soft-tissue rheumatism of the shoulder // Inflam. Res. 2001, V. 50, Suppl. 1:S24–29.
- Schoenfeld P. Gastrointestinal safety profile of meloxicam: a meta-analysis and systematic review of randomized controlled trials // Am J Med. 1999; 107(6A):48S–54S.
- Zeidler H., Kaltwasser JP, Leonard JP et al. Prescription and tolerance of meloxicam in day-to-day practice: postmarceting observational cohort study of 13307 patients // J. Clin. Rheum. 2002. V. 8. P. 305–315.
- Singh G., Triadafilopoulos G. Epidemiology of NSAIDs induced gastrointestinal complications // J. Rheum. 1999. V. 26 (Suppl. 56). P. 18–24.
- Martin RM, Biswas P., Mann RD The incidence of adverse events and risk factors for upper gastrointestinal disorders associated with meloxicam use amongst 19087 patients in general practice in England: cohort study // Br. J. Clin. Pharmacol. 2000, V. 50: 35–42.
- Winkelmeyer WC, Waikar SS, Mogun H., Solomon DH Nonselective and Cyclooxygenase-2-Selective NSAIDs and acute kidney injury // Am. J. Med. 2008, 121: 1092–1098.
- Bombardier C., Laine L., Reicin A. et al. “Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis // N. Engl. J. Med. 2000, 343: 1520–1528.
- Singh G. Meloxicam does not increase the risk of acute myocardial infarction, congestive heart failure, edema or hypertension compared to NSAIDs: results from a pooled analysis of 27039 patients [abstract] // Eur. Congress of Rheumatology. Prague, 2001, June 13–16.
- Altman R., Luciardi H.L., Muntaner J. et al. Efficacy assessment of meloxicam, a preferential cyclooxigenase-2 inhibitor, in acute coronary syndromes without ST-segment elevation: The Nonsteroidal Anti-Inflammatory Drugs in Instable Angina Treatment –2 (NUT-2) Pilot Study // Circulation. 2002, V. 196: 191–195.
- Lewis MF, Miller DR, Rahme E. et al. Pharmacoepidemiol Drug Saf. 2006; 15:S59.
- Singh G., Triadafilopoulos G. Meloxicam has a low risk of serious gastrointestinal complication: pooled analysis of 27039 patients: the results of the IMPROVE trial // EULAR Congress. 2001, Prague.
N. V. Chichasova , Doctor of Medical Sciences, Professor
Research Institute of Rheumatology RAMS , Moscow
Contact information about the author for correspondence
Note!
The description of the drug Meloxicam on this page is a simplified author’s version of the apteka911 website, created on the basis of the instructions for use.
Before purchasing or using the drug, you should consult your doctor and read the manufacturer's original instructions (attached to each package of the drug). Information about the drug is provided for informational purposes only and should not be used as a guide to self-medication. Only a doctor can decide to prescribe the drug, as well as determine the dose and methods of its use.
Contraindications and side effects
The drug should not be used if there are such contraindications:
- intolerance to pyrazolone and its derivatives;
- allergic reactions, hypersensitivity to acetylsalicylic acid;
- recurrent nasal polyposis;
- severe kidney damage;
- peptic ulcers of the gastrointestinal tract;
- bronchial asthma;
- period of pregnancy, as well as lactation (at any stage);
- older people take the drug with caution.
Meloxicam suppositories are not allowed to be used in the presence of diseases of the rectum, anal region, if they are chronic. Other contraindications are possible, so be sure to consult a doctor before starting use.
In some cases, side effects are observed:
- exacerbation of stomach and intestinal ulcers;
- vomiting, nausea;
- belching;
- bloating;
- constipation;
- diarrhea syndrome;
- headaches;
- dizziness;
- drowsy state;
- leukopenia;
- anemia;
- increased blood pressure;
- skin rashes, itching, other forms of allergies;
- swelling;
- high urea level;
- blood rush to the chest, face, neck.