The use of external agent Daivonex in combination with phototherapy in the treatment of psoriasis
P
soriasis, a disease that affects 1–3% of the world’s population, has been an ever-present medical and social problem since ancient times due to its widespread prevalence and modest treatment results [1–3].
Psoriasis is a chronic dermatosis of a multifactorial nature
with a dominant role in the development of genetic factors, characterized by hyperproliferation of epidermal cells;
disturbance of keratinization, inflammation in the dermis, probably supported by T-cell immune reactions [4–5]. The cardinal problem of psoriasis is the treatment of patients, especially its severe forms. World and domestic experience shows that all existing methods of treating psoriasis are insufficiently effective, take a long time, and cause side effects. Against this background, phototherapy methods have gained universal recognition as highly effective and accessible among all others [6–8]. Selection of the most effective and at the same time adequate therapy for a patient with psoriasis is the main task of the attending physician
.
The purpose of this clinical study was to determine the effectiveness and tolerability of the drug "Davonex"
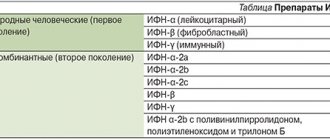
(“Daivonex”/Leo Pharmaceutical Products/Denmark) in combination with phototherapy. Contraindications to the use of this drug are: hypersensitivity to calcipotriol and impaired calcium metabolism in the body. The active substance of Daivonex is calcipotriol, a synthetic analogue of 1,25-dihydroxycholecalciferol (1,25 (OH)2 – D3), the most active metabolite of vitamin D3. By interacting with specific receptors in keratinocytes, calcipotriol causes a dose-dependent inhibition of the proliferation of these skin cells and accelerates their morphological differentiation. In addition, calcipotriol may block specific immune mediators, in particular interleukin 1, which are believed to play a significant role in the pathogenesis of psoriasis [9–11]. This may be a significant additional factor in the mechanism of action of this drug. Unlike other vitamin D3 analogues, calcipotriol has less effect on calcium metabolism in the body. Due to the characteristics of the dosage form, it penetrates the skin poorly (absorption is less than 1%), which also prevents the development of systemic side effects. Daivonex is available in three forms: lotion, cream, ointment.
Daivonex was applied 1–2 times a day in a thin layer to the affected areas of the skin, and in half of the cases alternating cream and ointment was used. The maximum daily dose did not exceed 15.0, and the weekly dose did not exceed 100.0 of cream or ointment. For lesions of the scalp, Daivonex was used in the form of a lotion. PUVA therapy was carried out according to the scheme 3–4 times a week, the initial dose of UVA rays was 0.5–1.0 J/cm2 with a gradual increase in dose by 0.25–0.5 J/cm2 for each subsequent procedure; 11–20 procedures per course. Ammifurin was used as a photosensitizer. SFT was carried out according to the same scheme, the initial dose of UVB rays was 0.05 J/cm2, increasing through the procedure by 0.05 J/cm2. The course included 12–18 procedures.
Under our supervision there were 40 patients with vulgar psoriasis in stationary, regressing stages, aged from 22 to 68 years, of which 14 were women and 26 men. The duration of the disease in patients ranged from 3 to 30 years, and 70% had a history of frequent exacerbations of the process (more than 2 times a year). A relatively common form was diagnosed in 60% of patients. In 30% of patients the process was accompanied by itching. In the past, all these patients used corticosteroid ointments as external therapy. With clinical recovery, 26 patients (65%) experienced complete resolution of all elements by the end of 4 weeks. In 14 patients (35%), positive therapeutic results (ie, clinical recovery or significant improvement) were observed by the end of 6 weeks. Intolerance or any other side effects were not observed when using the external agent Daivonex in patients with psoriasis. Also, no negative changes were noted in peripheral blood and biochemical parameters. The results of treatment with Daivonex are very encouraging
. Remission in most patients turned out to be long-term and stable (more than 10 months); withdrawal syndrome was not observed in any case. The use of calcipotriol allowed patients to refuse the use of external corticosteroid drugs.
Thus, the combination of phototherapy (PUVA therapy or SFT) with external use of the drug Daivonex increases the therapeutic effect and reduces the duration of treatment. Daivonex is highly effective and is a “first-line” drug in the treatment of vulgar psoriasis in stationary and regressive stages.
conclusions
1. The combination of phototherapy with external use of Daivonex increases the therapeutic effect and reduces the duration of treatment.
2. When combining Daivonex with PUVA therapy or SFT, the total radiation dose is reduced.
3. The drug Daivonex is highly effective and is a “first-line” drug for the treatment of vulgar psoriasis in stationary and regressive stages.
4. The external agent Daivonex is available in three forms (lotion, cream, ointment), which makes it convenient to use.
Literature:
1. Skripkin Yu.K., Skin and venereal diseases. no matter. – M.: Medicine, 1995. – vol. 2. – 543 p.
2. Fedorov S.M.. Psoriasis: clinical. and therapeutic aspects//Rus. honey. magazine 2000.–№11.–pp.447–450.
3. Drew GS Psoriasis//Prim. Care.–2000 – Vol.23, No. 2. – p.385–406.
4. Khobeish M.M., Moshkalova I.A., Sokolovsky E.V. Psoriasis – modern methods of treatment. – 1999. p. 70–132.
5. Vladimirov V.V., Menshikov L.V. Modern ideas about psoriasis and methods of its treatment. Rus. honey. magazine – 1998; vol. 6, no. 20 (80) p. 1318–1323.
6. Selvaag E.// J. Eur. Acad.Dermatol. – 2000 – Vol. 14, No. 1 – p. 10–21.
7. Rutter A. et al.// Hautarts.–2000–No. 6. – p.431–433.
8. Vladimirov V.V. // Vestn. Dermatol.–1983.–№7– p.8–12
9. Molin L.// J. of Dermatological Treatment. – 1997 – 8, p.261–263.
10. Molin L. et al.//Dermatology – 1999; 198; p.375 – 381.
11. Dubertred J. et al // J. Amer. Acad. Dermatol. – 1992. – Vol. 27. – p.983 – 988
Daivonex lotion instructions for use
Indications for use
The product is developed for the treatment of non-pustular psoriasis - chronic stable psoriasis vulgaris. It is also called ordinary, simple, plaque or vulgar. It also helps with psoriasis of the scalp (under the hair).
pharmachologic effect
Calcipotriol slows down the proliferation of skin cells. In addition, it promotes the division of keratinocytes. In other words, the lotion suppresses excessive cell formation and prevents them from combining into plaques. As a result of such treatment, a person’s lesions are significantly reduced (in some cases, even completely disappearing) and the skin acquires its normal color and appearance (swelling and redness go away).
Note! Daivonex does not cure the disease completely (it is believed that psoriasis cannot be cured 100% yet - this is the specifics of the problem). But it gives excellent results in terms of improving skin condition and controlling symptoms (long-term remission).
How to take, course of administration and dosage
This remedy can be used ONLY after consultation with your doctor!
The lotion is applied to the affected areas of the skin 2 times a day - it is best to do this in the morning and evening. The maximum permissible dose per week is 60 ml. During treatment, prolonged exposure to the sun should be avoided, and exposure to artificial lighting that simulates sunlight should be limited.
Note! It is not recommended to apply the lotion to the skin of the face, and after applying the drug you should wash your hands so that it does not accidentally get on your face.
Contraindications
The product is contraindicated for people with individual intolerance to one or more components included in the composition. It is strictly prohibited for those who suffer from diseases that cause calcium metabolism disorders.
It is undesirable (or very careful) to use Dayvonex lotion if you have the following problems:
- nephrolithiasis;
- hypercalciuria;
- hypervitaminosis D;
- hypercalcemia.
This is due to the fact that there is still no reliable information about the results of psoriasis treatment in people with such problems. For the same reason, the lotion should not be used by children under 6 years of age and elderly people over 65 years of age.
It is not advisable for pregnant women to use Daivonex, since the effect of calcipotriol on the female body and fetus in these cases has not yet been studied. It is also unknown whether this substance passes into breast milk or not.
It is not advisable to use the product simultaneously with drugs that contain salicylic acid.
Side effects
The most common side effects (approximately 1 in 100 people) include local skin irritation, itching, burning sensation, severe redness or rash (various types). Sometimes an exacerbation of the disease, as well as the appearance of dermatitis, is possible.
It is extremely rare that the use of lotion causes an allergic reaction, reversible depigmentation or hyperpigmentation, swelling around the eyes, or swelling of the face. Even less commonly, the drug can cause metabolic imbalance and provoke hypercalciuria or hypercalcemia. The latter is usually provoked by increasing the weekly dose of calcipotriol.
Storage conditions
Store the drug in tightly closed packaging out of the reach of children. Avoid exposure to sunlight. The optimal storage temperature is up to +25C. The shelf life is 3 years from the date of issue.
Daivonex
Trade name: Daivonex International name: Calcipotriol Formulations: cream for external use 50 mcg/g (aluminum tubes) 30, 100 g Composition: calcipotriol hydrate 52.2 mcg [equiv. 50 mcg calcipotriol] - 1 g, calcipotriol monohydrate 52.2 mcg [equiv. 50 mcg calcipotriol] - 1 g Dispensed without a doctor's prescription Shelf life: 2 years Registration data: P No. 016256/03 dated October 22, 2007, May 27, 2005
Pharmacological group: psoriasis treatment Pharmacological group according to ATK: D05AX02. Calcipotriol Pharmacological action: antipsoriatic Pharmacodynamics: Analogue of the active metabolite of natural vitamin D3. Causes dose-dependent inhibition of keratinocyte proliferation and accelerates their morphological differentiation. It has a slight effect on Ca2+ metabolism in the body (according to experimental data, 100-200 times less compared to calcitriol). The effect develops within 1-3 weeks. Pharmacokinetics: Absorption of the drug when applied to the skin depends on the dosage form and the condition of the skin. When applying a cream, systemic absorption is significantly lower than when using an ointment, from which up to 6% (± 3%) of the used dose in patients with psoriasis enters the systemic circulation. No more than 5% (± 2.6%) is absorbed from the surface of intact skin. When using the solution for external use, no more than 1% is absorbed. The affinity of calcipotriene for plasma proteins in vitro is 30 times less than calcitriol. Metabolized in the liver to form 2 inactive metabolites. Inactivated within 24 hours. Less than 1% is excreted in urine and feces. Indications: Vulgar psoriasis (including the scalp) in a stationary and regressive stage. Dosage regimen: Externally, ointment or cream is applied in a thin layer to the affected areas of the skin 2 times a day (after using the drug, you should avoid wearing compressive clothing). The maximum daily dose is 15 g, weekly dose is 100 g. The course of treatment for cream should not exceed 6-8 weeks, ointment - 1 year (due to the lack of longer clinical trials). The solution is applied to the affected areas of the scalp 2 times a day and rub lightly. Weekly dose - 60 ml. If the solution is used simultaneously with a cream or ointment, the total dose should not exceed 5 mg/week (60 ml of solution and 30 g of cream or ointment, or 30 ml of solution and 60 g of cream or ointment). Side effects: Dermatitis (skin itching, swelling and hyperemia of the skin) - in 10-15% of cases for ointment and cream and 1-5% for solution, skin rash - in 1-10% of cases for ointment and cream and 11% for solution , transient burning, skin irritation, skin itching - in 20% of cases for ointment and cream and 23% for solution, dryness and peeling of the skin - in 1-10% of cases for ointment and cream and 1-5% of cases for solution, erythema - in 1-10% of cases for ointment, exacerbation of psoriasis (including on the face and scalp) - in 1-10% of cases for ointment and cream and in 1-5% of cases for solution, rarely (in 1% cases for ointment and no effect for cream and solution), skin atrophy, folliculitis, hyperpigmentation - in less than 1% of cases for ointment and cream and no effect for solution, systemic effects (usually asymptomatic, self-limiting). Overdose. Symptoms (more than 100 g of ointment or cream in 1 week): hypercalcemia, abdominal pain, constipation, depression, unusual fatigue, increased blood pressure, loss of appetite, weight loss, thirst, nausea, vomiting, hypercalciuria. Treatment: discontinuation of the drug, administration of intensive hydration (oral and parenteral) of the body and forced diuresis (against the background of furosemide to increase the rate of drug excretion), potassium chloride (to prevent hypokalemia), corticosteroids, hemodialysis (for renal failure), monitoring of electrolytes, control daily diuresis. Contraindications: Hypersensitivity, psoriasis of the scalp in the acute stage, pregnancy, lactation. Interaction: It is not recommended to apply simultaneously with salicylic acid and preparations containing it. Special instructions: Should not be applied to large surfaces of lesions (more than 30% of the skin surface) and to the face. It is not recommended to use on large skin surfaces or in severe chronic forms of psoriasis (due to increased absorption and an increased risk of hypercalcemia). If there is a need for use in these forms of psoriasis, then careful monitoring of the concentration of Ca2+ in the blood and urine is required. Treatment continues as needed, depending on the effect, but not more than 1 year. Treatment can be combined with selective and PUVA phototherapy. Prescription to patients under 18 years of age is possible only in short courses and on a skin surface not exceeding 30% of the total body surface, and in cases where the prescription of other drugs does not lead to satisfactory results. Children are more at risk for systemic effects than adults (due to their higher ratio of skin surface area to body weight). Systemic effects are usually reversible and are leveled off after discontinuation of the drug. After applying to the affected area, you should wash your hands thoroughly to avoid getting the drug on your face , mucous membranes or healthy areas of the skin. Before treatment and regularly during therapy, it is recommended to monitor the concentration of Ca2+ in the blood. With continuous long-term use of large doses, the risk of developing hypercalcemia and focal calcifications in the kidneys cannot be completely excluded. Use of the drug during pregnancy is not recommended due to lack of sufficient clinical data on the use of the drug in pregnant women. In experiments on animals, fetotoxic phenomena were revealed: incomplete ossification of the pubic bone, phalanges of the upper limbs, ribs, cleft fontanel. It is not known whether calcipotriol is excreted in breast milk, therefore, if it is necessary to prescribe the drug during lactation, it is necessary to decide on stopping breastfeeding. Carefully. Hypercalcemia, hypercalciuria (including predisposition), vitamin D hypervitaminosis, history of nephrourolithiasis, age under 18 years, old age (over 65 years). Category of action on the fetus. C