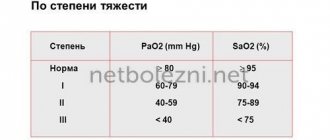
The action of Ivermectin and the features of its composition
The instructions indicate the following components of the medication:
- tocopherol acetate;
- ivermectin;
- benzyl alcohol;
- injection water;
- dimethylacetamide;
- polyethylene glycol 660-hydroxystearate.
Ivermectin helps fight lice, scabies, nematoses, and onchocerciasis. The medicine can be used orally, injected or topically. The drug is based on avermectins - insecticides used to create medicines against helminths and insect bait.
Once in the body, the solution is quickly absorbed. The maximum concentration in the blood is recorded after 4 hours, then it is evenly distributed throughout the body and excreted after 48 hours along with feces.
The action of the drug is based on blocking the neuromuscular impulses of parasites, which leads to their death. Provided that the dosage of the drug is observed when taking it, its toxicity is at the lower limits.
Drug interactions
Taking anti-parasite medications with drugs that increase GABA energy activity is prohibited. These medications include barbiturates, valproic acid, and benzodiazepines. Warfarin increases the amount of anthelmintic drug in a person's blood. After using corticosteroids, taking tablets is allowed no earlier than 21 days later. An antiparasitic agent for animals should not be combined with the use of macrocyclic lactones. The consequence of taking it may be an increase in the toxic effect of Ivermek.
Permissions and prohibitions for use from the instructions for Ivermectin
With the help of the drug it is treated:
- ascariasis;
- onchocerciasis;
- strongyloidiasis;
- trichocephalosis;
- filariasis;
- enterobiasis.
Ivermectin can be used for head lice and scabies, provided that the immune system is normally functioning.
The drug is contraindicated in patients:
- with body weight less than 15 kg and age under 5 years;
- pregnancy or breastfeeding;
- dysfunction of the liver, kidneys;
- individual intolerance to the component composition;
- insufficient functionality of the immune system;
- meningitis, bronchial asthma.
Before starting therapy, the patient should read the instructions for the medication and make sure that it has no contraindications. Violation of the rules can cause non-standard reactions of the body and will require long-term rehabilitation treatment.
Analogs
Preparations for animals, similar in composition, have some features. Analogues of Ivermek include the following:
- Iversect - contains an anesthetic.
- Ivomek – low cost.
- Baymek – shelf life 5 years.
- Ivertin – shelf life 3 years.
- Aversect K&S – recommended for dogs and cats.
- Ivermag is a complete analogue.
- Ganamectin – shelf life 4 years;
- Novomek – storage temperature up to +30 degrees.
Analogues of Ivermectin for humans
There are many drugs whose active ingredient is ivermectin. There are practically no complete analogues. In Russia, drugs similar in composition and spectrum of effects include the following:
- Ivermicol is an aqueous analogue.
- Ivermek-gel for external use.
- Ivervexan is an injection solution against pathogenic microorganisms.
- Vormil is the cheapest antiparasitic drug.
- Cesol is effective, but has serious side effects.
- Cysticide – identical to Ivermectin.
Adverse reactions
Responses associated with therapeutic procedures are based on depression of the nervous system. Side effects from Ivermectin include:
- severe drowsiness;
- dizziness;
- deterioration of health;
- problems with concentration;
- short-term fainting;
- disturbances in the functionality of the brain.
Sometimes there is dysfunction of the gastrointestinal tract, decreased visual acuity and enlargement of individual lymph nodes. The appearance of a non-standard response to treatment requires stopping it and selecting a more suitable medication.
Nuances of Ivermectin therapy
The instructions indicate that the most suitable use of the medicine is oral. A single dosage is 12 mg, but it is better to select the volume of medication according to the patient’s weight. The attending physician determines the number of procedures, subject to a mandatory break of 7-14 days. For children weighing more than 15 kg and over 5 years old, the dosage is calculated according to the standard: for each kilogram of weight, 150 mcg.
The drug is used to combat certain parasitic pathologies, including helminthiases, scabies, myiases, etc. It allows oral or injection administration using 2 or more doses and observing a break of 7-14 days. If there are superficial areas of damage, the medication is applied directly to them.
Medicines with the same active ingredient are also used in veterinary practice to treat pets. To combat parasites, the solution is prescribed for external treatment or oral administration. The dosage is calculated according to standard parameters: for each kilogram of the animal’s body weight, 300 mcg of the substance is taken.
Introduction
Ivermectin is a broad-spectrum parasiticide for systemic use, discovered in 1970 and used in medicine since 1987 in oral form.
The parasites experienced paralysis and death due to blocking of chemical transmission and nerve impulse transmission through γ-aminobutyric acid channels. In December 2014, the US Food and Drug Administration approved ivermectin 1% cream for the treatment of rosacea in adults. The drug was approved in Europe in March 2015. Ivermectin cream is a new, effective and well-tolerated treatment for inflammatory papulopustular rosacea in adults. The rationale for treating rosacea with ivermectin is based on its anti-inflammatory properties and antiparasitic effects. Ivermectin suppressed lipopolysaccharide-induced production of inflammatory cytokines (interleukin 1 (IL-1) and 6 (IL-6), tumor necrosis factor α (TNF-α)) in vitro in a mouse study, and this was mediated at least in part by suppression nuclear factor kB pathway. Ivermectin has also been shown to inhibit multiple proinflammatory cytokines that are downstream of the LL-37 activation chain, including IL-6 and TNF-α.
However, the effect of ivermectin on IL-8, a pro-inflammatory cytokine responsible for neutrophil recruitment, remains to be elucidated. A recent study showed that ivermectin is able to inhibit kallikrein 5 (KLK5) and LL-37 gene expression and protein secretion in calcitriol-stimulated normal human epidermal keratinocytes (NHEKs). The anti-inflammatory effects of ivermectin were associated with inhibition of the secretion of IL-8, IL-6 and MCP-1 (CCL2) from NHEK cells. These results indicate that ivermectin can prevent the inflammatory effects of rosacea caused by LL-37 by inhibiting KLK5 gene expression in the epidermis.
Azelaic acid, commonly used in the treatment of rosacea, perioral dermatitis and acne, has the ability to directly inhibit the expression of KLK5 and cathelicidin genes. Ivermectin has a similar structure to macrolide antibiotics, but its use is not associated with the development of antibiotic resistance. Topical ivermectin 1% cream is indicated for the treatment of rosacea.
We searched the following databases up to March 2021: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, SCOPUS and Science Citation Index and concluded that there is one report in the literature of the possible use of topical ivermectin in the treatment of perioral dermatitis and there are no reports of other inflammatory facial dermatoses: seborrheic dermatitis and acne vulgaris. Despite the different pathophysiological mechanisms of development of these disorders, we decided to use symptomatic treatment, suggesting the potential anti-inflammatory activity of ivermectin.
Treatment of perioral dermatitis can be difficult in children and adults. Demodex mites, although part of the normal skin microbiome, are more abundant in rosacea and perioral dermatitis than in healthy skin. In contrast to rosacea and perioral dermatitis, Demodex folliculorum colonization in acne and seborrheic dermatitis is comparable to the healthy population. Given the increase in antibiotic resistance in patients with acne, new treatment options should be evaluated. The mechanism of action of topical ivermectin should also be effective in reducing the inflammatory lesions of acne as well as seborrheic dermatitis.
Target
The aim of our study was to evaluate the benefit and tolerability of topical ivermectin therapy for mild to moderate perioral, seborrheic dermatitis and acne.
Materials and methods
We present 20 cases of various facial inflammatory dermatoses (8 cases of perioral dermatitis, 8 cases of seborrheic dermatitis and 4 cases of papulopustular acne) treated in our centers between November 2021 and May 2017. All patients, 16 women and 4 men, were aged 18 years or older (range 18 to 54 years at diagnosis, mean age: 32.4 years), disease duration was 2-36 months (mean duration 21.56 ± 1 ,11 months). Ivermectin 1% cream in oil was administered once daily for 1 to 3 months depending on clinical response.
results
All patients responded very well to treatment with topical ivermectin. During the study period, a gradual decrease in inflammatory skin lesions was observed. Complete or almost complete resolution was achieved in 8 cases with perioral dermatitis. The onset of the therapeutic effect could be observed already 2 weeks after the start of treatment with 1% ivermectin cream.
For perioral dermatitis, it took 4 patients 4 weeks, 1 patient 5 weeks, 2 patients 6 weeks, and 1 patient 12 weeks to achieve complete cure. Of the 8 patients with seborrheic dermatitis, 4 achieved complete regression of the rash after 4 weeks of therapy and another 4 patients after 6 weeks. In acne patients, it took 8 weeks (1 patient) and 10 weeks (3 patients) to achieve complete remission.
The results of treatment with 1% ivermectin cream were also very satisfactory for patients in the study group. Overall, 19 patients reported “very good” and “excellent” improvement (8 with perioral dermatitis, 8 with seborrheic dermatitis, and 3 with acne). Only 1 patient with acne rated the treatment results as “good.” We did not observe disease recurrence during the 18-week follow-up period in any of our patients.
Discussion
The overall safety and effectiveness of topical treatment of perioral, seborrheic dermatitis and acne with ivermectin 1% cream make this drug potentially attractive for use in these skin conditions.
First, ivermectin is able to suppress the production of the inflammatory mediators nitric oxide (NO) and prostaglandin E2 (PGE2) and reduce the expression levels of inducible NO synthase (iNOS) and cyclooxygenase-2 (COX2) mRNA by inhibiting phosphorylation of p38 mitogen-activated protein kinases (MAPKs). , extracellular signal-regulated kinase (ERK)N1/2 and C-Jun N-terminal kinase (JNK). Inhibition of NO and PGE2 production by ivermectin results from inhibition of iNOS and COX2 gene expression.
Secondly, the anti-inflammatory effects of topical ivermectin have been demonstrated in clinical trials by reducing the number of inflammatory elements of the rash. This drug may cause burning, itching, and dryness in 0.7% to 1.8% of patients, which is consistent with our results. The onset of the therapeutic effect can be observed already 2 weeks after the start of monotherapy with 1% ivermectin cream. The absence of the risk of systemic accumulation of this cream after 52 weeks of use allows you to plan the duration of treatment individually for each patient.
Based on the mode of action and results obtained in our studies, topical ivermectin appears to be effective in the treatment of mild to moderate perioral dermatitis, seborrheic dermatitis, and acne. The effectiveness of ivermectin in perioral dermatitis was recently demonstrated by Noguera-Morel et al. The antiparasitic activity of topical ivermectin was examined in a clinical study evaluating the safety and effectiveness of three lotion concentrations of this drug in the treatment of head lice. A single application of a 0.5% concentration of this ivermectin lotion was most effective in eliminating head lice in the majority of subjects.
Miyajima et al. A new method of administering ivermectin for the treatment of scabies using whole body bathing has been proposed. The results showed that this method could deliver ivermectin to the human stratum corneum without systemic exposure or serious adverse events in healthy volunteers and at concentrations adequate for the treatment of scabies. There are no published data in the literature on the treatment of acne and seborrheic dermatitis using topical and systemic ivermectin.
Based on the documented anti-inflammatory properties of ivermectin, we decided to introduce the therapy on a case-by-case basis in patients with these two conditions. Our patients with seborrheic dermatitis did not have a satisfactory response to conventional treatment (topical imidazoles, calcineurin inhibitors and corticosteroids) with frequent exacerbations.
Patients with acne were treated for 2–3 years (consecutively) with topical medications (retinoids, benzoyl peroxide, antibiotics) with transient remission and refused systemic treatment. All patients with acne included in the study had papulopustular and nodular acne with a relatively small number of comedones. Interestingly, patients with seborrheic and perioral dermatitis achieved improvement over a shorter period of time (4-6 weeks) than patients with acne (8-10 weeks). The only exception was a patient with perioral dermatitis who responded to treatment after 12 weeks.
We hypothesized that the positive response to therapy in patients with acne could be achieved through the potential anti-inflammatory mechanism of action of ivermectin without any additional direct antimicrobial effect. This would likely explain the longer duration of improvement in patients with acne compared with patients with perioral dermatitis, but the small number of patients treated did not allow definite conclusions to be drawn.
One of the biggest therapeutic problems in patients with perioral, seborrheic dermatitis and acne is the tendency to relapse and short-term remission. The well-documented effectiveness of ivermectin 1% cream for papulopustular rosacea in randomized trials at 12–16 weeks was confirmed in a 36-week study assessing the duration of remission and time of first relapse in patients treated with ivermectin and metronidazole 0.75% cream.
The study found that initial successful treatment with ivermectin significantly prolonged the remission of rosacea compared with initial treatment with metronidazole after discontinuation of treatment. The mean duration of remission was significantly longer (115 days vs. 85 days) and the relapse rate at the end of the study period was significantly lower (62.7% vs. 68.4%) for patients initially successfully treated with ivermectin than with metronidazole. The mean number of treatment-free days was longer with ivermectin compared with metronidazole (196 days vs. 169.5 days). Interestingly, we did not observe relapse in our patients at 18 weeks of follow-up, but a longer period of time is obviously needed to compare results.
Our pilot study has several limitations. The study sample (20 patients) was small and the follow-up time after achieving remission was insufficient. A well-designed prospective study with a larger number of patients is needed to confirm our results.
Overdose symptoms and manufacturer's instructions
Taking large amounts of Ivermectin can lead to intoxication of the body. Help with an overdose consists of gastric lavage and taking activated charcoal. In difficult cases, symptomatic therapy is carried out based on the symptoms that appear.
The instructions recommend paying attention to the following nuances:
- simultaneous use with Warfarin can increase the content of active components in the bloodstream;
- combination with other inhibitors increases the risk of crossing the blood-brain barrier, which will provoke serious adverse reactions;
- Individual intolerance to the component composition can lead to allergies; if unusual symptoms appear, the patient should take antihistamines and report the problem to the attending physician.
Drinking alcoholic beverages during therapeutic procedures may cause side effects. During treatment, drinking any drinks containing ethanol is strictly prohibited.
Rules for storing the drug and its sale
The drug requires a doctor's prescription when purchased at a pharmacy. Ivermectin is not sold in the Russian Federation, the medicine comes from abroad, the price when ordering varies from 25 to 50 US dollars.
The medication is stored in its original packaging in darkened rooms. The medicine should not come into contact with food and is safe from small children and pets. The solution is good for 2 years, after opening it must be used within 42 days. After the expiration date, the medicine is disposed of with household waste.
Indications for use
The drug is widely used in the treatment of infections caused by roundworms, for example, for:
- onchocerciasis;
- strongyloidiasis;
- ascariasis;
- trichocephalosis;
- filariasis;
- enterobiasis.
In addition, it can be used in the treatment of scabies and lice , if people do not have weakened immune defenses.