Nalidixic acid
Name: Nalidixic acid (Acidumnalidixicum) Pharmacological action: It is a synthetic antibacterial drug. Effective against infections caused by gram-negative bacteria, intestinal, dysentery and typhoid bacilli, Proteus (a type of microorganism that, under certain conditions, can cause infectious diseases of the small intestine and stomach), Klebsiella bacillus (Friedlander - bacteria that cause pneumonia and local purulent processes). It acts bacteriostatically and bactericidally (prevents the proliferation and destroys bacteria). Effective against strains resistant to antibiotics and sulfonamides. It is ineffective against gram-positive cocci (staphylococci, streptococci, pneumococci) and pathogenic anaerobes (bacteria that can exist in the absence of oxygen and cause human diseases). The drug is well absorbed from the gastrointestinal tract. About 80% is excreted unchanged in the urine. The half-life (the time during which 1/2 the dose of the drug is eliminated) is approximately 8 hours, but in case of renal failure it reaches 20 hours or more.
Indications for use: Used mainly for urinary tract infections: cystitis (inflammation of the bladder), pyelitis (inflammation of the renal pelvis), pyelonephritis (inflammation of the kidney tissue and renal pelvis) - caused by microorganisms sensitive to the drug. Most effective for acute infections. It is also prescribed for the prevention of infections during operations on the kidneys and bladder. Recommended for enterocolitis (inflammation of the small and large intestines), cholecystitis (inflammation of the gallbladder), inflammation of the middle ear and other diseases caused by microorganisms sensitive to the drug, including those resistant to other antibacterial agents.
Directions for use: Adults take 0.5 g orally (1 capsule or 1 tablet), and for more severe infections - 1 g 4 times a day. The course of treatment is at least 7 days. For long-term treatment, use 0.5 g 4 times a day. Children are prescribed at the rate of 60 mg/kg, dividing the daily dose into 4 equal parts.
Side effects: Nalidixic acid is generally well tolerated; however, nausea, vomiting, diarrhea, headache, and dizziness are possible. Allergic reactions may occur (dermatitis /skin inflammation/, increased body temperature, eosinophilia /increased number of eosinophils in the blood/), as well as increased skin sensitivity to sunlight (photodermatoses). In patients with cerebrovascular accidents, parkinsonism, and epilepsy, seizures may occur. Due to the possibility of convulsive reactions, one should beware of an overdose of the drug in children. Severe adverse reactions require temporary or complete discontinuation of the drug.
Contraindications: Impaired liver function, depression of the respiratory center. Great care is required in case of insufficient renal function. It should not be prescribed to women in the first 3 months. pregnancy and children under 2 years of age. The drug should not be used simultaneously with nitrofurans, as this reduces the antibacterial effect.
Release form: In capsules or tablets of 0.5 g.
Storage conditions: List B. In a dry place, protected from light.
Synonyms: Nevigramon, Negram, Cystidix, Nagram, Nalidin, Nilidixan, Nalidixin, Naligram, Nalix, Nalurin, Naxuril, Nogram, Notricel, Specifin, Urodixin, Urogram, Uroneg, Vintomilon.
Attention! The description of the drug “ Nalidixic acid ” on this page is a simplified and expanded version of the official instructions for use. Before purchasing or using the drug, you should consult your doctor and read the instructions approved by the manufacturer. Information about the drug is provided for informational purposes only and should not be used as a guide to self-medication. Only a doctor can decide to prescribe the drug, as well as determine the dose and methods of its use.
Nevigramon
INSTRUCTIONS for medical use of the drug Nevigramon (NEVIGRAMON (R))
General characteristics: international and chemical names: Nalidixic acid - 1-ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid; basic physical and chemical properties: opaque hard gelatin capsules with yellow body and cap 1 tablet contains 500 mg of nalidixic acid. Excipients: aqueous silicon dioxide, stearic acid, yellow quinoline, sunset yellow, titanium dioxide, gelatin. Release form. Capsules. Pharmacological group. Uroantisetics and antimicrobial agents. Quinolone derivatives. PBX code J01MB02.
Pharmacotherapeutic properties. Pharmacological. Nalidixic acid has pronounced antibacterial activity against gram-positive bacteria, including Proteus mirabilis, P. morganii, P. vulgaris, P. rettgeri, Escherichia coli, Enterobacter (Aerobacter), Klebsiella. Pseudomonas strains are usually resistant to the drug. Nalidixic acid acts by selectively inhibiting bacterial DNA synthesis. Nalidixic acid has a bactericidal effect at any pH level. At low concentrations, nalidixic acid affects only proliferating microorganisms by inhibiting DNA replication. With prolonged exposure, it also inhibits bacterial synthesis of RNA and protein. The minimum inhibitory concentration (MIC) of nalidixic acid is 5-75 mcg/ml. At higher concentrations, the bactericidal effect of nalidixic acid occurs through the disintegration of DNA molecules. Pharmacokinetics. Nalidixic acid is rapidly absorbed from the gastrointestinal tract, partially metabolized in the liver and quickly excreted by the kidneys. Altered nalidixic acid is detected in urine along with its active metabolite, hydroxy-nalidixic acid, which has the same antibacterial activity as nalidixic acid. Hydroxynalidixic acid makes up 30% of the biologically active drug in the blood and 85% in the urine. Maximum serum active drug levels average 20-50 mcg/mL 2 hours after fasting administration of 1 g of nalidixic acid in healthy individuals. The half-life is 6-7 hours. About 93% of nalidixoic acid and 63% of hydroxy-nalidixic acid are bound to plasma proteins. The maximum level of active drug in urine after taking a single dose of 1 g averages about 150-250 mcg/ml. Alkalinization of urine increases the concentration of unchanged drug in the urine. Approximately 4% of nalidixic acid is excreted in the feces. Nalidixic acid crosses the placenta and small amounts appear in breast milk. Indications. Treatment of urinary tract infections caused by gram-negative microorganisms sensitive to nalidixoic acid. Treatment of bacterial dysentery. Method of administration and dose. Nevigramon is recommended to be taken on an empty stomach, preferably an hour before meals. Adults. The average dose is 4 g (2 capsules 4 times a day) for at least 7 days. If the use of the drug must be continued, the dose can be reduced to 1 capsule 4 times a day. Children over 12 years old (weighing more than 40 kg). The recommended daily dose is 50 mg/kg body weight (4 times 1 capsule). Patients with renal failure. For patients with a creatinine clearance of 20 ml/min and lower, the dose is reduced by half (see “Special Instructions”).
Side effect. From the central nervous system, drowsiness, headache, and dizziness may occur. In rare cases - toxic psychosis, short-term convulsions. Convulsions are usually observed after an overdose in patients with epilepsy and cerebral arteriosclerosis. Palsy of the sixth cranial nerve, which quickly resolves after discontinuation of the drug. Vision. Rarely, reversible subjective visual impairment may occur (usually within the first few days of taking the drug after each dose): a feeling of excessive brightness of light, color perception, difficulty focusing, decreased visual acuity, diplopia. Usually these phenomena disappear quickly after reducing the dose or discontinuing the drug. From the gastrointestinal tract. Abdominal pain, nausea, vomiting, diarrhea. Allergic reactions. Rash, itching, urticaria, eosinophilia, arthralgia with joint stiffness and swelling. Rarely - angioneurotic swelling, anaphylactic shock and anaphylactoid reactions. From the side of the skin. Photosensitivity reactions: erythema and blisters on the exposed skin surface, which usually resolve completely within two weeks to two months after discontinuation of nevigramon. Blisters may continue to appear with exposure to sunlight or minor damage to the surface of the skin for up to three months after stopping the drug. Other. Rarely - cholestasis, paresthesia, metabolic acidosis, thrombocytopenia, leukopenia or hemolytic anemia, sometimes accompanied by glucose-6-phosphate dehydrogenase deficiency. Contraindications. Hypersensitivity to nalidixic acid and its related compounds. History of convulsions, Parkinson's disease, severe cerebral arteriosclerosis. Kidney and liver failure. Glucose-6-phosphate dehydrogenase deficiency. Porphyria. Children under 12 years old. First trimester of pregnancy. Lactation.
Overdose. Patients who have taken more than the recommended dose may experience toxic psychosis, convulsions, increased intracranial pressure or metabolic acidosis, nausea, vomiting and lethargy. These phenomena are short-term (diabo 3:00), since the drug is quickly eliminated from the body. If the drug is already absorbed, increased fluid intake is recommended; It is necessary to have oxygen and artificial respiration available. In very severe cases, anticonvulsant therapy may be required.
Features of application. Pre-pubertal period. Nevigramon causes erosions in the cartilage of weight-bearing joints and other signs of arthropathy in most animals of the studied species that have reached sexual maturity. Therefore, Nevigramon should be prescribed with caution during puberty. If arthralgia is detected, use of Nevigramon should be discontinued. Caution should be exercised when prescribing nevigramon to patients suffering from liver disease (see “Side Effects”) and severe renal failure. Such patients may require a dose reduction (see "Dosage and Administration"). If it is necessary to use nevigramon for more than two weeks, it is recommended to conduct a blood test and monitor liver and kidney function. Patients should be warned to avoid excessive exposure to sunlight; if photosensitivity reactions occur, Nevigramonuslide should be discontinued. If the patient develops symptoms of increased intracranial pressure, psychosis or other toxic effects, the use of Nevigramon should be discontinued. If the use of nevigramon does not give the expected result, it is necessary to conduct a microorganism sensitivity test. Bacterial resistance to Nevigramon usually develops within the first 48 hours of drug use. Cross-resistance has been observed between nevigramone and other quinolol derivatives such as oxolinic acid and cinoxacin.
The effect on the ability to drive a car and perform work that requires increased attention is insignificant.
Pregnancy. The safety of using nevigramon during pregnancy has not been established. Therefore, the drug can be used during pregnancy only when the expected benefit outweighs the potential risk, especially during the first trimester of pregnancy (Nevigramon penetrates the placental barrier and, as studied in animals, is embedded in cartilage tissue and develops) and in the last month pregnancy, as the baby may have high levels of the drug in the blood.
Lactation. The drug passes into breast milk, so administration of Nevigramon during lactation is contraindicated.
Interaction with other drugs. Nevigramon can enhance the effect of oral anticoagulants such as warfarin or bis-hydroxycoumarin - nevigramon occupies a significant number of their binding sites with plasma proteins. With the simultaneous use of nevigramon and oral anticoagulants, it is necessary to monitor the vatiprothrombi time or INR (international normalizes whose indicator), it may be necessary to change the dose of the anticoagulant. Since the antibacterial effect of Nevigramon requires the proliferation of bacterial cells, its effect can be suppressed in the presence of other antibacterial agents, especially bacteriostatic ones, such as tetracycline, chloramphenicol or nitrofurantoin (the latter is an antagonist of Nevigramon in vitro). Probenecid inhibits the secretion of nevigramone in the renal tubules and may reduce its effectiveness against genitourinary tract infections, thereby increasing the development of systemic side effects. The simultaneous use of Nevigramon and melphalan is associated with gastrointestinal toxicity. Laboratory test results. If methods based on copper reduction are used to analyze the urine of patients taking Nevigramon (for example, the use of Benedict's or Fehling's solutions), an erroneous positive reaction to glucose may be obtained. Therefore, it is recommended to use specific methods based on glucose oxidase. Incorrect values may be obtained when determining 17-keto and ketogenic steroids in urine when performing tests based on vimirvanillyl mandelic acid in urine. In such cases, it is recommended to use the Porter-Silber test for 17-hydrocorticosteroids.
Storage conditions. Store at room temperature (from 15 ° C to + 25 ° C), protected from light. Shelf life: 5 years.
Gemifloxacin: 10 years of clinical use in the treatment of lower respiratory tract infections
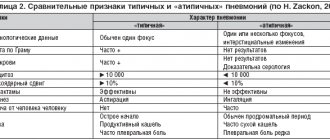
The history of the birth of the class of quinolone antibiotics dates back to the 1960s, when nalidixic acid was obtained through the synthesis of chloroquine (Fig. 1). For two decades, nalidixic acid and its derivatives (pipemidic and oxolinic acids), which have activity against gram-negative microorganisms, have been successfully used to treat urinary tract infections. The second wave of development of quinolones (1980s) is associated with the emergence of fluorinated compounds with higher activity against gram-negative and gram-positive bacteria, intracellular microorganisms with improved pharmacokinetics, the presence of forms for parenteral administration (ciprofloxacin, ofloxacin, fleroxacin, lomefloxacin, norfloxacin) [2]. The next stage in the development of quinolones (1990s) is associated with the emergence of di- and trifluorinated compounds with enhanced activity against gram-positive bacteria (especially Streptococcus pneumoniae) and intracellular pathogens. This quality determined the name of these drugs - “respiratory” fluoroquinolones, which, according to the modern classification, belong to the III (sparfloxacin, levofloxacin) and IV (moxifloxacin, gatifloxacin, garenoxacin) generations of quinolones. In 1999, a research group from the LG Life Sciences laboratory (South Korea) synthesized a new drug classified as the fourth generation of quinolone antibiotics by adding an aminomethyl group at the C7 position of the pyrrolidine ring (Fig. 2). As it turned out, this formula provided the unique properties of gemifloxacin, which is characterized by the greatest antipneumococcal activity among all representatives of this class of antimicrobial drugs. The mechanism of action of gemifloxacin is bactericidal and is similar to the mechanism of action of other fluoroquinolones. It is based on disruption of the processes of replication, repair and transcription of bacterial DNA through inhibition of the enzymes DNA gyrase (topoisomerase II) and topoisomerase IV, which are necessary for bacterial growth. A distinctive feature of “respiratory” fluoroquinolones is their high effectiveness against all potential pathogens of CAP. At the same time, gemifloxacin, as already noted, has the highest activity against S. pneumoniae. This has been demonstrated in a number of studies, according to the results of which its minimum inhibitory concentration for 90% of the studied strains (MIC90) against pneumococcus is 0.015–0.03 μg/ml [5–7]. For levofloxacin this indicator is 1–2 μg/ml, for moxifloxacin it is 0.12–0.25 μg/ml (Table 1). Gemifloxacin is also active against S. pneumoniae resistant to penicillin and macrolides, and most strains resistant to ofloxacin and levofloxacin. The activity of gemifloxacin against “atypical” pathogens (Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila) is comparable to the activity of other “respiratory” fluoroquinolones. The main mechanism for the development of resistance to fluoroquinolones is mutations in the DNA gyrase and DNA topoisomerase IV genes. It is important that for classical fluoroquinolones, for the development of resistance, one mutation of the genes encoding one or another subunit of topoisomerase IV may be sufficient, while for “respiratory” fluoroquinolones additional mutations in the DNA gyrase genes are required. The use of gemifloxacin, due to the greatest pneumococcal activity, is characterized by a minimal probability of single and double sequential mutation, which was shown in an experimental model of pneumococcal pneumonia [9]. Resistance of pneumococcus to “respiratory” fluoroquinolones throughout the world, with the exception of a number of countries in Southeast Asia, remains low [10]. In Europe, more than 97% of S. pneumoniae strains are sensitive to “respiratory” fluoroquinolones [11]; in Russia, according to the results of the PeGAS-III study (Table 2), there are no pneumococcal strains resistant to levo-, moxi- and gemifloxacin [12]. Strains of Haemophilus influenzae resistant to “respiratory” fluoroquinolones have also not been isolated in the Russian Federation. All “respiratory” fluoroquinolones have a long half-life, which allows them to be taken once a day, and are characterized by high bioavailability and rapid absorption. The antimicrobial effect of fluoroquinolones depends on the antibiotic concentrations created, with the best pharmacodynamic parameter correlating with bacterial eradication being the ratio of AUC (non-protein bound fraction of the antibiotic) to MIC. A reliable predictor of S. pneumoniae eradication is a free AUC/MIC ratio of ≥25. For levofloxacin, moxifloxacin and gemifloxacin, this indicator is 40, 96 and 97–127, respectively (Table 3) [7, 13]. “Respiratory” fluoroquinolones have good tissue penetration, creating concentrations in alveolar macrophages, bronchial mucosa and the fluid lining the epithelium of the respiratory tract that significantly exceed the MIC of pathogens of respiratory infections that are sensitive to them. Gemifloxacin is characterized by better penetration than other fluoroquinolones into alveolar macrophages and bronchial mucosa (Table 4). Elimination of the antibiotic occurs predominantly through the intestines (>60%), and only 27–30% of gemifloxacin is excreted unchanged from the body through the kidneys, i.e. Elimination of the antibiotic occurs primarily through non-renal pathways. However, in patients with severe renal impairment (creatinine clearance ≤29 ml/min), dose adjustment of gemifloxacin is necessary. The drug is not metabolized by the cytochrome P450 system, which determines the absence of clinically significant drug interactions, in particular with theophylline, warfarin, digoxin, and oral contraceptives. Eating practically does not change the pharmacokinetics of the drug, so it can be taken regardless of food. It is worth noting that when concurrently prescribing ferrous sulfate or sucralfate to a patient, they must be taken 4 hours before or 2 hours after taking gemifloxacin. Considering the fact that the clinical use of some fluoroquinolone antibiotics (grepafloxacin, trovafloxacin, clinafloxacin, etc.) was accompanied by a high incidence of serious adverse events (AEs), “respiratory” fluoroquinolones are under close attention of researchers and practitioners. However, a sufficient number of studies and a long period of use of these drugs in clinical practice allow us to note that levofloxacin, moxifloxacin and gemifloxacin are characterized by a favorable safety profile. In a comparative review, P. Ball et al. The safety of gemifloxacin was analyzed in comparison with other antibacterial drugs used in the treatment of respiratory tract infections (Table 5) [14]. The incidence of serious AEs with gemifloxacin was comparable to the incidence of AEs with macrolides, β-lactams, and other fluoroquinolones. The main problem with gemifloxacin was the appearance of maculopapular skin rash with long-term courses of its use in women under 40 years of age and postmenopausal women on hormone replacement therapy. In this regard, its use in this category of patients in courses of more than 7 days is not recommended. The frequency of other AEs (nausea, diarrhea, headache, etc.) was comparable. A moderate and reversible increase in the concentration of hepatic transaminases, exceeding 1.5–3 times the upper limit of normal, is observed in 1–5% of patients receiving therapy with “respiratory” fluoroquinolones. At the same time, serious hepatotoxic adverse reactions with the use of levo-, moxi- and gemifloxacin are rare [15]. On the contrary, with regard to severe liver damage, the example of trovafloxacin is indicative. It was with its use that unpredictable reactions occurred, leading in some cases to death or requiring liver transplantation. The incidence of severe hepatic reactions with trovafloxacin averages 58 per 10 million prescriptions, and therefore the license for its use has been revoked. At the same time, according to the FDA, the incidence of severe liver damage when using moxifloxacin and levofloxacin does not exceed 6.6 and 2.1 cases per 10 million prescriptions, respectively. There are no data on such cases when taking gemifloxacin. All fluoroquinolones are, to one degree or another, capable of influencing the QT interval, which is a group property of this group of antimicrobial drugs (Table 6) [16]. Thus, grepafloxacin was withdrawn from the pharmaceutical market based on 3 reports of non-fatal torsade de pointes ventricular tachycardia and 7 reports of deaths due to cardiac disorders, the connection of which with the drug was regarded as possible. Sparfloxacin has the most pronounced negative effect on the cardiac conduction system; clinical manifestations of cardiotoxicity have also been recorded with the use of ofloxacin, gatifloxacin and other fluoroquinolones. It should be noted that in almost all cases, clinically significant prolongation of the QT interval when taking fluoroquinolones was observed in patients with risk factors (drug interactions with other drugs that prolong the QT interval, female gender, older age, concomitant cardiac pathology, genetic predisposition and electrolyte disturbances). Given the possible risk of this complication, “respiratory” fluoroquinolones are not recommended for use in patients with congenital or acquired prolongation of the QT interval; with hypokalemia; with clinically significant bradycardia; with clinically significant heart failure with reduced ejection fraction; with a history of ventricular arrhythmias. The drugs should not be used concomitantly with other drugs that can prolong the QT interval, class IA antiarrhythmic drugs (quinidine, procainamide) or class III (amiodarone, sotalol). The high clinical efficacy of gemifloxacin for lower respiratory tract infections (CAP, ACP, chronic obstructive pulmonary disease (COPD)) has been proven in a number of clinical studies. Noteworthy is the fact that in almost all studies the use of gemifloxacin was compared with treatment regimens using parenteral antibiotics. Thus, N. Lode et al. (2002) demonstrated comparable clinical and bacteriological effectiveness of oral monotherapy with gemifloxacin and combination stepwise therapy (ceftriaxone → cefuroxime ± macrolide) in the treatment of hospitalized patients with CAP [17]. Both treatment regimens showed comparable high clinical efficacy - 92.2% in the group receiving gemifloxacin, and 93.4% in patients receiving stepwise treatment (ceftriaxone → cefuroxime ± macrolide), as well as bacteriological efficiency, which was 92 and 89.9 % respectively. In a multicenter, double-blind study that included 571 patients with non-severe CAP, gemifloxacin, administered 320 mg once a day orally for 7–14 days (2/3 of patients received an antibiotic for only 7 days), demonstrated high clinical efficacy - 95. 8%, comparable to the effectiveness of trovafloxacin, used 200 mg 1 time / day orally in the treatment of CAP - 93.6% [18]. In a study by P. Leophonte et al. (2002) studied the effectiveness and safety of gemifloxacin (320 mg 1 time/day orally for 7 days) in comparison with high-dose therapy with amoxicillin/clavulanate (1000/125 mg 3 times/day orally for 10 days) in the treatment of patients with suspected pneumococcal CAP [19]. Both treatment regimens were effective both at the end of therapy (95.3 and 90.1%, respectively) and 21–28 days after the start of the study - 88.7% (gemifloxacin) and 87.6% (amoxicillin/clavulanate) . The bacteriological effectiveness of gemifloxacin and amoxicillin/clavulanate after completion of therapy was 96.3 and 91.8%, respectively. In a Russian study, gemifloxacin demonstrated high clinical (96.7%) and bacteriological (83.4%) efficacy in the treatment of hospitalized patients with CAP, which was comparable to comparison therapy (amoxicillin/clavulanate IV followed by switching to amoxicillin/clavulanate orally ± clarithromycin) [20]. In a study by R. Wilson et al. (2003) studied the effectiveness of gemifloxacin in comparison with ceftriaxone → cefuroxime therapy in patients with exacerbation of COPD (Anthonisen type I), requiring treatment in a hospital setting [21]. It turned out that the use of oral gemifloxacin demonstrates high clinical efficacy - 92% (analysis on days 2-4 after the end of therapy), superior to the effectiveness of parenteral use of ceftriaxone with a transition to oral cefuroxime - 88.2% (ITT population). At the same time, the duration of the hospital period when using gemifloxacin was significantly less than in the comparison group. The GLOBE trial assessed the comparative effectiveness and safety of gemifloxacin (320 mg/day for 5 days) and clarithromycin (1000 mg/day for 7 days) in patients with exacerbation of COPD. The authors showed that taking gemifloxacin was characterized by a significant reduction in the incidence of subsequent exacerbations compared to taking clarithromycin [22]. And finally, we currently have the results of a meta-analysis performed by Chinese scientists and including 10 randomized clinical trials (RCTs) examining the comparative effectiveness of gemifloxacin in the treatment of CAP and ACH/COPD (3940 patients) (Table 7) [23]. Five studies compared gemifloxacin with other quinolones (levofloxacin, trovafloxacin). 5 RCTs compared gemifloxacin with β-lactams and/or macrolides (amoxicillin/clavulanate, clarithromycin, ceftriaxone (IV)/cefuroxime (per os). When analyzing the effectiveness of treatment regimens, it was shown that gemifloxacin demonstrates higher therapeutic efficacy in treating patients both CAP and exacerbation of COPD than comparator antibiotics (Fig. 3). All-cause mortality during the study period was assessed in 8 RCTs. There was no significant difference between gemifloxacin and comparator antibiotics for this indicator. 9 RCTs assessed microbiological efficacy. According to the meta-analysis, there was no significant difference between gemifloxacin and comparator antibiotics. There were also no significant differences between comparator antibiotics in the eradication of S. pneumoniae (182 isolates), H. influenzae (160 strains), M. catarrhalis (90) and atypical pathogens (M. pneumoniae, C. pneumoniae and Legionella spp.) Compared with other fluoroquinolones (Fig. 4) gemifloxacin demonstrates higher clinical efficacy, while no significant differences were observed between β-lactams and macrolides (Fig. 5). It is worth noting that the lack of differences is likely due to the use in a number of RCTs of parenteral forms of cephalosporin antibiotics and high-dose therapy with “protected” aminopenicillins. Data on AEs possibly related to antibiotic therapy were obtained from all RCTs included in the meta-analysis. There were no significant differences between gemifloxacin and other quinolones in the overall incidence of AEs (Fig. 6). It was noted that in patients who received gemifloxacin, AEs such as diarrhea and rash occurred somewhat more often. When compared with β-lactams and/or macrolides, gemifloxacin was associated with a lower incidence of side effects. Regarding the occurrence of maculopapular rash, the administration of gemifloxacin was accompanied by a higher incidence of this AE, but no statistically significant difference was obtained. Concluding this review, I would like to note that more than 10 years of successful experience in the effective and safe clinical use of gemifloxacin, a number of evidence of its effectiveness, including the cited meta-analysis, have allowed gemifloxacin to occupy an important place in modern regimens of antibacterial therapy for CAP and ACBP/COPD. Thus, in national guidelines for the management of patients with CAP, gemifloxacin, along with levofloxacin and moxifloxacin, is recommended for use in patients with non-severe pneumonia who have risk factors1 for therapeutic failure [29]. “Respiratory” fluoroquinolones (levofloxacin, moxifloxacin and gemifloxacin) are the drugs of choice for complicated2 ACB/COPD [30]. 1 Presence of concomitant diseases: COPD, diabetes mellitus, congestive heart failure, chronic renal failure, liver cirrhosis, chronic alcoholism, drug addiction, exhaustion and/or use in the last 3 months. antimicrobials for more than 2 days. 2 Presence of ≥1 characteristic: age ≥60 years; FEV1<50%; ≥4 exacerbations per year; accompanying illnesses; oxygen therapy at home; long-term use of steroids by mouth; hospitalization for a previous exacerbation of COPD in the past 12 months.
References 1. US FDA panel backing for Factive. SCRIP 2003; 2830: 22. Electronic resource https://www.antibiotic.ru/index.php?article=174. 2. Anderson M., MacGowan A. Development of the quinolones // JAC. 2003. Vol. 51. Suppl. 1. R. 1–11. 3. Ball P. Quinolone generations: natural history or natural selection? J. Antimicrob. Chemother. 2000. Vol. 46. R. 17–24. 4. Chang Yong Hong. Discovery of gemifloxacin (Factive, LB2o304a): a quinolone of a new generation // Farmaco. 2001. Vol. 56. R. 41–44. 5. Hoban D., Bouchillon S., Johnson J. et al. Comparative in vitro activity of gemifloxacin, ciprofloxacin, levofloxacin and ofloxacin in a North American surveillance study. Diagn Microb Infect Dis. 2001. Vol. 40. R. 51–57. 6. Koeth L., Jacobs M., Bajaksouzian S. et al. Comparative in vitro activity of gemifloxacin to other fluoroquinolones and non-quinolone agents against Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis in Unified States in 1999-2000 // Intern J Antimicrob Agents. 2002. Vol. 19. R. 33–37. 7. Blondeau J., Missaghi B. Gemifloxacin: a new fluoroquinolone // Expert Opin Pharmacother. 2004. Vol. 5. R. 1117–1152. 8. Hoshino K., Inoue K., Murakami Yo. et al. In Vitro and In Vivo Antibacterial Activities of DC–159a, a New Fluoroquinolone // Antimicrobial Agents and Chemotherapy. 2008. Vol. 52 (1) R. 65–76. 9. Sinopalnikov A.I. Gemifloxacin in the treatment of lower respiratory tract infections in adults // Clinical microbiology and antimicrobial chemotherapy. 2006. No. 3. T. 8. P. 260–269. 10. Kozlov R.S., Veselov A.V. Respiratory fluoroquinolones / Community-acquired respiratory tract infections: diagnosis and treatment. M.: M-Vesti LLC, 2008. pp. 88–105. 11. Jones M., Draghi D., Thornsberry C., Sahm D. A current perspective on S. pneumoniae and H. influenzae resistance trends in Europe: GLOBAL Surveillance Study, 2005. Proceedings of 16th ECCMID, 2006. Abst.rp. 1629. 12. Kozlov R.S., Sivaya O.V., Krechikova O.I., Ivanchik N.V. et al. Dynamics of Streptococcus pneumoniae resistance to antibiotics in Russia for the period 1999–2009. (Results of a multicenter prospective study of PeGAS) // Clinical microbiology and antimicrobial chemotherapy. 2010. No. 12 (4). pp. 329–341. 13. Zanel G., Noreddin A. Pharmacokinetics and pharmacodynamics of the new fluoroquinolones: focus on respiratory infections // Curr Opin Pharmacol. 2001. Vol. 1. R. 459–463. 14. Ball P., Mandell L., Patou G. et al. A new respiratory fluoroquinolone, oral gemifloxacin: a safety profile in context // Int J Antimicrob Agents. 2004. Vol. 23. R. 421–429. 15. Leitner J., Graninger W., Thalhammer F. Hepatotoxicity of antibacterials: Pathomechanisms and clinical // Infection. 2010. Vol. 38(1). R. 3–11. 16. Iannini P. Quinolone-induced QT interval prolongation: a not-so-unexpected class effect // J Antimicrob Chemother. 2001. Vol. 47 (6). R. 893–894. 17. Lode H., File TM, Mandell L. et al. Oral gemifloxacin versus sequential therapy with intravenosus ceftriaxone/oral cefuroxime with or without a macrolide in the treatment of patients hospitalized with community-acquired pneumoniae: a randomized, open-label, multicenter study of clinical efficacy and tolerability // Clin Therapeut. 2002. Vol. 24. R. 1915–1936. 18. File T., Schlemmer B., Garau J. et al. Efficacy and safety of gemifloxacin in the treatment of community-acquired pneumonia: a randomized, double-blind comparisoin with trovafloxacin // JAC. 2001. Vol. 48. R. 67–74. 19. Leophonte P., File TM, Feldman C. Gemifloxacin once daily for 7 days compared to amoxicillin/clavulanic acid thrice daily for 10 days for the treatment of community-acquired pneumonia of suspected pneumococcal origin // Respir Med. 2004. Vol. 98. R. 708–720. 20. Sinopalnikov A.I., Zaitsev A.A. Gemifloxacin: new possibilities for antibacterial therapy of community-acquired pneumonia in adults // Russian Medical News. 2007. No. 1. P. 4–12. 21. Wilson R., Langan C., Ball P. et al. Oral gemifloxacin once daily for 5 days compared with sequential therapy with iv ceftriaxone/oral cefuroxime (maximum of 10 days) in the treatment of hospitalized patients with acute exacerbations of chronic bronchitis // Respir Med. 2003. Vol. 97. R. 242–249. 22. Wilson R., Schentage JJ, Ball P. et al. A comparison of gemifloxacin and clarithromycin in acute exacerbations of chronic bronchitis and long-term clinical outcomes // Clin Therapeut. 2002. Vol. 24. R. 639–652. 23. Zhang L., Wang R., Matthew F., Chen L., Liu Y. Gemifloxacin for the treatment of community-acquired pneumonia and acute exacerbation of chronic bronchitis: a meta-analysis of randomized controlled trials // Chin Med J (Engl). 2012. Vol. 125(4). R. 687–695. 24. Yao Z. A study of the effectiveness of gemifloxacin on chronic obstructive pulmonary disease // PractClin Med. 2008. Vol. 9. R. 36–37. 25. Shi H., Huang Z., Huang Y. et al. Gemifloxacin versus levofloxacin in the treatment of lower respiratory tract infection: a randomized controlled trial // Chin J New Drugs Clin Rem (Chin). 2007 Vol. 26. R. 678–680. 26. Sethi S., Fogarty C., Fulambarker A. A randomized, double-blind study comparing 5 days oral gemifloxacin with 7 days oral levofloxacin in patients with acute exacerbation of chronic bronchitis // Respir Med. 2004. Vol. 98. R. 697–707. 27. Ball P., Wilson R., Mandell L. et al. Efficacy of gemifloxacin in acute exacerbations of chronic bronchitis: a randomized, double-blind comparison with trovafloxacin // J Chemother. 2001. Vol. 13. R. 288–298. 28. File T., Schlemmer B., Garau J. et al. Gemifloxacin versus amoxicillin/clavulanate in the treatment of acute exacerbations of chronic bronchitis. The 070 Clinical Study group // J Chemother. 2000. Vol. 12. R. 314–325. 29. Chuchalin A.G., Sinopalnikov A.I., Kozlov R.S. et al. Community-acquired pneumonia in adults. Practical recommendations for diagnosis, treatment and prevention. M., 2010. 82 p. 30. Sinopalnikov A.I., Kozlov R.S., Romanovskikh A.G., Rachina S.A. Infectious exacerbation of COPD: practical recommendations for diagnosis, treatment and prevention // Russian medical news. 2006. Issue. XI (No. 1). pp. 4–18.