Tresiba FlexTouch injection solution 100 U/ml 3 ml syringe pen 5 pcs. in Moscow
PC,
Once a day at any time of the day, but it is preferable to administer the drug at the same time every day.
Tresiba® FlexTouch® is an ultra-long-acting insulin analogue.
In patients with type 2 diabetes mellitus, Tresiba® FlexTouch® can be used either as monotherapy or in combination with PGGP, GLP-1 receptor agonists, or bolus insulin (see “Pharmacodynamics”). Patients with type 1 diabetes mellitus are prescribed Tresiba® FlexTouch® in combination with short-acting/ultra-short-acting insulin to cover the need for prandial insulin.
The dose of Tresiba® FlexTouch® should be determined individually according to the needs of the patient. To optimize glycemic control, it is recommended to adjust the dose of the drug based on fasting plasma glucose levels.
As with any insulin preparation, dose adjustment of Tresiba® FlexTouch® may also be necessary if the patient increases physical activity, changes his usual diet, or has a concomitant disease.
Tresiba® FlexTouch® starting dose
Patients with type 2 diabetes mellitus.
The recommended initial daily dose of Tresiba® FlexTouch® is 10 units, followed by selection of an individual dose of the drug.
Patients with type 1 diabetes mellitus.
Tresiba® FlexTouch® is prescribed once a day in combination with prandial insulin, which is administered with meals, followed by selection of an individual dose of the drug.
Transfer from other insulin preparations
Careful monitoring of blood glucose concentrations is recommended during transfer and in the first weeks of prescribing a new drug. It may be necessary to adjust concomitant hypoglycemic therapy (dose and time of administration of short-acting and ultra-short-acting insulin or other simultaneously used hypoglycemic drugs).
Patients with type 2 diabetes mellitus.
When switching to Tresiba® FlexTouch® in patients with type 2 diabetes mellitus who are on a basal or basal-bolus insulin regimen, or a regimen of ready-mixed insulin/self-mixed insulin, the dose of Tresiba® FlexTouch® should be calculated based on the dose of basal insulin, that the patient received before switching to a new type of insulin, on a unit-by-unit basis, and then adjusted according to the individual needs of the patient.
Patients with type 1 diabetes mellitus.
For most patients with type 1 diabetes, when switching from any basal insulin to Tresiba® FlexTouch®, a unit-for-unit approach is used based on the dose of basal insulin the patient was receiving before switching, then the dose is adjusted to meet individual needs. In patients with type 1 diabetes mellitus who, at the time of transfer to therapy with Tresiba® FlexTouch®, were on insulin therapy with basal insulin administered twice daily, or in patients with an HbA1c <8%, the dose of Tresiba® FlexTouch® should be set on an individual basis. basis. It may be necessary to reduce the dose followed by individual selection based on glycemic parameters.
Use of Tresiba® in combination with GLP-1 receptor agonists in patients with type 2 diabetes mellitus.
When adding Tresiba® to treatment with GLP-1 receptor agonists, the recommended initial daily dose is 10 units, followed by individual dose adjustment.
When adding GLP-1 receptor agonists to treatment with Tresiba, it is recommended to reduce the dose of Tresiba by 20% to minimize the risk of hypoglycemia. Subsequently, the dose should be adjusted.
Flexible dosing regimen
Based on the needs of the patient, Tresiba® FlexTouch® allows you to change the time of its administration (see “Pharmacodynamics”). In this case, the interval between injections should be at least 8 hours. For those patients who forget to administer a dose of insulin on time, it is recommended to administer the dose as soon as they discover this, and then return to their usual time of daily single administration of the drug.
Tresiba® FlexTouch® 100 and 200 U/ml. Tresiba® FlexTouch® is available in two dosages. For both dosages, the required dose of the drug is set in units. However, the dose increments differ between the two dosages of Tresiba® FlexTouch®.
Tresiba® FlexTouch®, 100 U/ml allows you to administer doses from 1 to 80 U in 1-U increments in a single injection. Tresiba® FlexTouch®, 200 U/ml allows you to administer doses from 2 to 160 U in 2-U increments in a single injection. The dose of insulin is contained in half the volume of solution compared to basal insulin preparations of 100 U/ml.
The dose counter shows the number of units regardless of the dosage; there is no need to recalculate the dose when transferring patients to a new dosage.
Special patient groups
Elderly patients (over 65 years old).
Tresiba® FlexTouch® can be used in elderly patients. Blood glucose concentrations should be carefully monitored and the insulin dose adjusted individually (see Pharmacokinetics).
Failure of kidney and liver function.
Tresiba® FlexTouch® can be used in patients with impaired renal and hepatic function. Blood glucose concentrations should be carefully monitored and the insulin dose adjusted individually (see Pharmacokinetics).
Children and teenagers.
Tresiba® can be used to treat adolescents and children over 1 year of age (see Pharmacodynamics). When switching from basal insulin to Tresiba®, the need to reduce the dose of basal and bolus insulin should be considered in each individual case to minimize the risk of hypoglycemia (see “Special Instructions”).
Mode of application
Tresiba® FlexTouch® is intended for subcutaneous administration only. Tresiba® FlexTouch® cannot be administered intravenously, because this may lead to the development of severe hypoglycemia. Tresiba® FlexTouch® cannot be administered intramuscularly, because in this case, the absorption of the drug changes. Tresiba® FlexTouch® cannot be used in insulin pumps.
Tresiba® FlexTouch® is injected subcutaneously into the thigh, anterior abdominal wall or shoulder area. Injection sites within the same anatomical area should be rotated regularly to reduce the risk of developing lipodystrophy. Tresiba® FlexTouch® is a pre-filled pen designed for use with NovoFine® or NovoTwist® disposable injection needles.
Directions for use of the drug
FlexTouch® is a pre-filled pen designed for use with NovoFine® or NovoTwist® needles up to 8 mm in length.
Tresiba® FlexTouch®, 100 U/ml allows you to administer doses from 1 to 80 U in 1-U increments.
Tresiba® FlexTouch® 200 U/ml allows you to administer doses from 2 to 160 U in 2-U increments. It is necessary to strictly follow the instructions contained in the attached Instructions for use of the FlexTach® syringe pen.
Tresiba® FlexTouch® and needles are for individual use only. The pen cartridge must not be refilled. Do not use the drug if the solution is no longer transparent and colorless. Do not use the drug if it has been frozen. The needle should be thrown away after each injection.
Follow local regulations for disposal of used medical supplies.
Instructions for patients on the use of Tresiba® FlexTouch®, solution for subcutaneous administration 100 or 200 U/ml
You must read these instructions carefully before using the Tresiba® FlexTouch® Pre-Filled Pen. If the patient does not follow the instructions, he may inject too little or too much insulin, which could result in blood glucose levels that are too high or too low.
A pen should only be used after the patient has learned how to use it under the guidance of a doctor or nurse.
Check the label on your pen to make sure it contains Tresiba® FlexTouch®, and then carefully look at the illustrations below that show the parts of the pen and needle.
If the patient is visually impaired or has severe vision problems and cannot distinguish the numbers on the dose counter, the pen should not be used without assistance. This patient can be assisted by a person without visual impairments who has been trained in the proper use of the FlexTouch® pre-filled syringe pen.
Tresiba® FlexTouch® 100 units/ml is a pre-filled pen containing 300 units of insulin degludec. The maximum dose you can set is 80 units in 1 unit increments.
Tresiba® FlexTouch® 200 U/ml allows you to administer doses from 2 to 160 U in 2-U increments.
The Tresiba® FlexTouch® pen is designed for use with NovoFine® or NovoTwist® disposable needles up to 8 mm in length. Needles are not included in the package.
Important information
. Pay attention to the information marked with such icons, this is very important for the safe use of the pen.
Tresiba® FlexTouch® 100 U/ml and needle (example).
Figure 1. Tresiba® FlexTouch®, 100 U/ml.
Figure 2. Tresiba® FlexTouch®, 200 U/ml
I. Preparing the syringe pen for use
Check the name and dosage on the pen label to ensure it contains Tresiba® FlexTouch® 100 IU/mL/Tresiba® FlexTouch® 200 IU/mL. This is especially important if the patient uses different types of insulin. If he injects another type of insulin by mistake, the blood glucose concentration may be too high or low.
A. Remove the cap from the syringe pen
Figure A.
B. Make sure that the insulin preparation in the syringe pen is transparent and colorless. Look at the insulin balance scale window. If the drug is cloudy, the pen syringe cannot be used.
Figure B
.
C. Take a new disposable needle and remove the protective sticker
Figure C.
D. Place the needle on the pen and turn it so that the needle fits tightly onto the pen.
Figure D
E. Remove the outer needle cap, but do not throw it away. You will need it after the injection is complete to safely remove the needle.
Figure E.
F. Remove and discard the inner needle cap. If the patient tries to put the inner cap back on the needle, they may stick themselves.
A drop of insulin may appear at the end of the needle. This is normal, but the patient should still check their insulin supply.
Figure F.
Important information.
Use a new needle for each injection to avoid contamination, infection, insulin leakage, needle blockage, and injecting the wrong dose of medication. Never use a needle if it is bent or damaged.
II. Checking insulin delivery
G. Before each injection, check the insulin supply. This way the patient can be sure that the full dose of insulin has been administered. Dial 2 units of the drug by turning the dose selector. Make sure the dose counter shows “2”.
Figure G.
H. Holding the pen with the needle pointing up, lightly tap the top of the pen several times with your fingertip to force air bubbles upward.
Figure H.
I. Press the start button and hold it in this position until the dose counter returns to zero. “0” should be opposite the dose indicator. A drop of insulin should appear at the end of the needle. Sometimes a small air bubble may remain at the end of the needle, but it will not be introduced when injecting. If a drop of insulin does not appear at the end of the needle, repeat operations IIG–II I, but no more than 6 times. If a drop of insulin does not appear, change the needle and repeat operations IIG–II I again.
Figure I.
If a drop of insulin does not appear at the end of the needle, do not use this syringe pen, use a new syringe pen.
Important information.
Before each injection, make sure that a drop of insulin appears at the end of the needle. This ensures the supply of insulin. If no insulin drop appears, no dose will be given even if the dosage counter moves. This may indicate that the needle is blocked or damaged.
Before each injection, check the insulin supply. If a patient does not check their insulin supply, they may inject insufficient or no insulin, which can result in blood glucose levels that are too high.
III. Setting the dose
J. Before starting the injection, make sure that the dose counter is set to the “0” position. “0” should be opposite the dosage indicator. Rotate the dose selector to set the dose prescribed by the doctor. The dose selector sets the number of units. Only the dose counter and dose indicator show the number of units of insulin in the selected dose. If the wrong dose is set, the patient can turn the dose selector forward or backward until the correct dose is set. The maximum dose he can set is 80/160 units. If the remaining insulin in the pen is less than 80/160 units, the dose counter will stop at the number of insulin units remaining in the pen. Each time you turn the dose selector, clicks are heard; the sound of the clicks depends on which direction the dose selector is rotated (forward, backward, or if the dialed dose exceeds the number of units of insulin remaining in the pen). Don't count these clicks.
Figure J.
Important information.
Before each injection, check how many units of insulin the patient has collected using the dose counter and dose indicator. Do not set the dose by the number of clicks. If the patient sets and administers the incorrect dose, the blood glucose concentration may become too high or low.
The insulin remaining scale shows the approximate amount of insulin remaining in the pen, so it cannot be used to measure insulin doses
IV. Insulin administration
K. Insert the needle under the skin using the injection technique recommended by your doctor. Make sure the dose counter is within the patient's field of vision. Do not touch the dose counter with your fingers as this may interrupt the injection. Press the start button all the way and hold it in this position until the dose counter shows “0”. “0” should be exactly opposite the dose indicator, and the patient may hear or feel a click.
After injection, leave the needle under the skin for at least 6 seconds. This will ensure that the full dose of insulin is administered.
Drawing K.
L. Remove the needle from under the skin by pulling the syringe pen upward.
If blood appears at the injection site, lightly press a cotton swab onto the injection site. Do not massage the injection site.
Figure L
After the injection is completed, the patient may see a drop of insulin at the end of the needle. This is normal and does not affect the dose of the drug that is administered.
Important information.
Always check the dose counter to ensure you know how many units of insulin have been administered. The dose counter will show the exact number of units. Don't count the number of clicks. Hold the start button until the dose counter shows “0”. If the dose counter stops before showing 0, the full dose of insulin has not been delivered, which may result in the blood glucose concentration being too high.
V. After completing the injection
M. Place the outer needle cap on a flat surface and insert the tip of the needle into the cap without touching it or the needle.
Drawing M.
N. When the needle enters the cap, carefully place the cap on the needle. Unscrew the needle and discard it, taking precautions.
Figure N.
A. After each injection, put a cap on the pen to protect the insulin it contains from exposure to light. Throw away the needle after each injection to avoid contamination, infection, insulin leakage, needle blockage, and injecting the wrong dose of medication. If the needle becomes blocked, the patient will not be able to inject insulin. Discard used pens with the needle removed as directed by your doctor, nurse, pharmacist, or local regulations.
Drawing O.
Important information.
To avoid accidental needle sticks, never attempt to put the inner cap back on the needle. Remove the needle from the pen after each injection and store the pen with the needle disconnected. This will avoid contamination, infection, insulin leakage, needle blockage, and incorrect dosage administration.
VI. How much insulin is left?
P. The insulin remaining scale shows the approximate amount of insulin remaining in the pen.
Figure P
R. To find out how much insulin is left in your pen, you need to use a dose counter. Rotate the dosage selector until the dose counter stops. If the dose counter shows 80/160, this means that there is at least 80/160 units of insulin left in the pen. If the dose counter shows less than 80/160, this means that the exact number of units of insulin left in the pen is the one displayed on the dose counter.
Rotate the dose selector in the opposite direction until the dose counter shows “0”. If the insulin remaining in the pen is not enough to administer the full dose, you can administer the required dose in two injections using two pens.
Figure R.
Important information.
You must be very careful when calculating the remaining required insulin dose. If the patient has doubts, it is better to inject himself with a full dose of insulin using a new syringe pen. If the patient makes a mistake in the calculations, he may enter an insufficient dose or too much insulin. This can cause your blood glucose concentration to become too high or low.
You should always carry a pen with you. You should always carry a spare pen and new needles with you in case they are lost or damaged. Keep the syringe pen and needles out of the reach of everyone, and especially children. Never share your syringe pen and its needles with others. This may lead to cross-infection. Caregivers should handle used needles with extreme care to avoid accidental sticking and infection.
Syringe pen care
Handle the syringe pen carefully. Careless or improper handling may result in incorrect dosing, which may result in glucose concentrations that are too high or too low.
Do not leave your pen in a car or any other place where it may be exposed to extremely hot or cold temperatures.
Protect the syringe pen from dust, dirt and all types of liquids.
Do not wash the pen, immerse it in liquid or lubricate it. If necessary, the pen can be cleaned with a damp cloth and a mild detergent. Do not drop or hit the pen on a hard surface. If the patient drops the pen or doubts its functionality, attach a new needle and check the flow of insulin before injecting.
Refilling the syringe pen is not allowed. An empty syringe pen should be thrown away immediately. Do not try to repair the syringe pen yourself or take it apart.
pharmachologic effect
The drug Tresiba FlexTouch is an analog of ultra-long-acting human insulin, produced by recombinant DNA biotechnology using the Saccharomyces cerevisiae strain.
Insulin degludec specifically binds to the human endogenous insulin receptor and, interacting with it, realizes its pharmacological effect similar to the effect of human insulin.
The hypoglycemic effect of insulin degludec is due to an increase in the utilization of glucose by tissues after insulin binds to receptors in muscle and fat cells and a simultaneous decrease in the rate of glucose production by the liver.
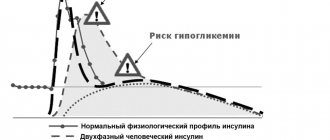
Tresiba FlexTouch is a basal analogue of ultra-long-acting human insulin; after subcutaneous injection, it forms soluble multihexamers in the subcutaneous depot, from where there is continuous and prolonged absorption of insulin degludec into the bloodstream, providing a long-term flat profile of action and a stable hypoglycemic effect of the drug. During a 24-hour period of monitoring the hypoglycemic effect of the drug in patients who were dosed with insulin degludec once a day, Tresiba FlexTouch, unlike insulin glargine, demonstrated a uniform Vd between the effects in the first and second 12-hour periods.
The duration of action of Tresiba FlexTouch is more than 42 hours within the therapeutic dose range. Css of the drug in blood plasma is achieved 2-3 days after administration of the drug.