Pharmacodynamics
The immunopharmacological mechanisms of action of the drug are that it activates the following parts of the immune defense:
— NK cells, which, 2–3 hours after exposure to the drug, intensely express CD69 activation molecules; the cytolytic activity of NK cells increases 3 times;
— circulating monocytes 2–4 hours after activation by the drug begin to secrete cytokines: interleukin-8, interleukin-1-beta and tumor necrosis factor alpha;
— neutrophil granulocytes are activated due to the activation of monocytes; the drug does not have a direct effect on neutrophil granulocytes; interleukin-8 secreted by monocytes causes activation of neutrophil granulocytes, which clearly manifests itself 24 hours after exposure to the drug;
— tissue macrophages, which manifests itself in changes in the morphology of these cells, increased production of bactericidal substances, changes in 5′-nucleotidase activity;
- formation of antibodies against foreign antigens, soluble and corpuscular.
Strengthens protection against infections caused by viruses (human papillomavirus, herpes simplex virus, parvovirus, canine distemper virus and others) or bacteria (Escherichia coli, salmonella, staphylococcus, chlamydia, mycoplasma, ureaplasma and others). This effect manifests itself in adults and newborns when the drug is administered in various ways: intramuscularly, intravenously, intraperitoneally, orally.
Immunomax lyophilisate intramuscularly 200 units n3 bottles with solvent
Indications: correction of weakened immunity;
—treatment of pathological conditions (condylomas, warts, dysplasia) caused by the human papillomavirus;
—infections caused by Herpes simplex, Chlamydia spp., Mycoplasma spp., Ureaplasma spp., other bacteria and viruses.
pharmachologic effect
Immunomodulator. Strengthens immune defense against viral and bacterial infections. The immunopharmacological mechanisms of action of the drug are that Immunomax activates the following parts of the immune system:
—NK cells that, 2-3 hours after Immunomax , intensely express CD69 activation molecules. The cytolytic activity of NK cells increases 3 times;
—circulating monocytes 2-4 hours after activation by Immunomax begin to secrete cytokines: interleukin-8, interleukin-1β and tumor necrosis factor alpha;
—neutrophil granulocytes are activated through monocytes; the drug does not have a direct effect on neutrophil granulocytes. Interleukin-8 secreted by monocytes causes activation of neutrophil granulocytes, which clearly manifests itself 24 hours after exposure to Immunomax ;
— tissue macrophages, which manifests itself in changes in the morphology of these cells, increased production of bactericidal substances, and changes in 5'-nucleotidase activity;
—formation of antibodies against foreign antigens, soluble and corpuscular.
Immunomax enhances immunological defense mechanisms in infections caused by viruses (human papillomavirus, herpes simplex virus, parvovirus, canine distemper virus) or bacteria (Escherichia coli, salmonella, staphylococcus, chlamydia, mycoplasma, ureaplasma). This effect of the drug manifests itself in adults and newborns when Immunomax in various ways: intramuscularly, intravenously, intraperitoneally, orally.
Pharmacokinetics
The pharmacokinetics of the drug have not been studied due to its peptidoglycan nature and very small effective doses.
Instructions for use / dosage
Recommended dose for adults
and
children aged 12 years and older
is 100-200 IU IM 1 time/day.
Before use, the contents of the bottle (ampoule) are dissolved in 1 ml of water and administered intramuscularly at 100-200 units, depending on the severity of the disease. Course of treatment – 6 injections on days 1, 2, 3, 8, 9, 10 of treatment.
For recurrent anogenital warts
a course of 6 injections of 200 IU
of Immunomax is combined with the destruction of warts (cryodestruction, electrocoagulation, laser destruction or the use of the drug Solcoderm).
For infections caused by bacteria or viruses
, conduct a course of 6 injections of 100-200 units
of Immunomax .
To correct weakened immunity
carry out a course of 3-6 injections of 100-200 units
of Immunomax .
Side effect
When used according to indications and in recommended doses, no side effects were detected.
Contraindications
— children under 12 years of age;
- hypersensitivity to the drug.
Use during pregnancy and breastfeeding
There is no data on the effects of Immunomax on pregnant women. As with other medicinal products, Immunomax should not be used during pregnancy unless the benefit to the patient outweighs the possible risk to the fetus.
Immunomax is not recommended for use by nursing mothers.
special instructions
The drug can be used as part of complex therapy according to indications.
Overdose
To date, no cases of overdose of Immunomax have been reported.
Drug interactions
Drug interactions with Immunomax have not been described.
Storage conditions and periods
The drug should be stored at a temperature of 4° to 8°C. Shelf life – 2 years.
Conditions for dispensing from pharmacies
The drug is available with a prescription.
Directions for use and doses
V/m. The recommended dose for adults and children over 12 years of age is 100–200 units once a day. Before use, the contents of the ampoule are dissolved in 1 ml of water for injection, 100–200 units are administered intramuscularly, depending on the severity of the disease. The course of treatment is 6 injections on days 1, 2, 3, 8, 9, 10 of treatment.
Treatment of recurrent anogenital warts: a course of 6 injections of 200 units combined with the destruction of warts using one of the generally accepted methods: cryodestruction, electrocoagulation, laser destruction or the prescription of solcoderm. Treatment of infections caused by bacteria or viruses: a course of 6 injections of 100–200 units. Correction of weakened immunity: a course of 3–6 injections of 100–200 units.
"Immunomax" in the treatment of recurrent genital papillomavirus infection
G
genital infection caused by the human papillomavirus (HPV) is one of the most common sexually transmitted diseases. The maximum incidence occurs in the age group from 18 to 28 years, regardless of gender and age [2,3]. Currently, approximately 100 serotypes of papillomaviruses are known, approximately 30 of them affect the anogenital area [3,4]. The human papillomavirus specifically infects the superficial layer of the skin and mucous membranes, causing condylomatous atypia of the epithelium. Malignant degeneration of the epithelium is usually associated with viruses of high and low oncogenic risk [5]. The close relationship with precancerous lesions of the genitals is acquiring social significance at the present stage, especially for young people [1].
None of the existing treatment methods ensures the complete disappearance of warts, and therefore the problem of recurrence
infections. Approximately 25% of patients report relapses within 3 months after treatment [2]. The level of relapse is often associated not with reinfection, but with the reactivation of the pathogenic virus, which induces the search for effective drugs with pronounced antiviral activity [1].
This study presents the results of the use of a new domestic drug " Immunomax"
» in the treatment of recurrent anogenital warts. The drug Immunomax® (Vitan) belongs to the group of immunomodulators and is an acidic peptidoglycan isolated from plants. The immunomodulatory effect of the drug is to strengthen the mechanisms of protection against bacterial and viral infections by activating important parts of the immune system. Immunomax is able to enhance the cytolytic activity of NK cells and tissue macrophages, activate the secretion of proinflammatory cytokines by circulating monocytes, and also intensify the process of producing antibodies against foreign antigens. The drug is produced by Immapharma LLC (Russia) in the form of lyophilized powder in bottles of 200 units. Immunomax is indicated for use in persons with weakened immune systems, as well as for the treatment of infections caused by bacteria and viruses.
Materials and research methods
The study involved 30 patients (14 women, 16 men) with a manifest form of human papillomavirus infection aged 18 to 46 years. Anogenital warts were found in all 30 subjects, with a history of multiple attempts to treat the disease. The duration of the disease with recurrent anogenital warts ranged from 3 to 180 months. (average duration 12 months). The relapse rate varied from 4 to 20 per year (average 11 relapses per year). On admission, most patients had no complaints. Only 14 complained of itching (n=8), burning (n=5), discomfort in the affected area (n=8). During an objective examination, anogenital warts were found in 8 patients - on the vaginal mucosa, in 8 - on the skin of the genitals, in 5 - in the anus, in 3 - on the back and head of the penis, in 3 - on the inner layer of the foreskin, in 6 - on the coronal sulcus.
Along with papillomavirus infection, recurrent genital herpes was found as a concomitant disease in 7 patients, urethritis in 5, bronchial asthma in 1, atopic dermatitis in 2, endocervicitis in 1, vaginal candidiasis in 8.
Cytological examination of smears was carried out in all patients, which made it possible to determine the severity of the inflammatory process and the nature of the microflora. The presence of the following infections was also determined by direct immunofluorescence: Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma hominis, Candida albicans, Herpes simplex virus, Cytomegalovirus
.
In all 30 patients, the content of human papillomavirus in material taken from the lesions was semi-quantitatively determined by polymerase chain reaction (PCR). At the same time, we took into account what type of virus it was, whether it was highly oncogenic (types 16,18,31,33,35,45,56) or low-oncogenic (types 6,11,42,43,44). PCR results were assessed in terms of HPV counts from + to +++. All patients involved in this clinical study had massive infection with human papillomavirus. When using PCR diagnostics, a significant amount of human papillomavirus was detected.
Table 1 shows the distribution of patients by the number and type of human papillomavirus detected by PCR.
Cytological examination of smears (Table 2) made it possible to determine the intensity of the inflammatory process and the nature of the microflora that caused inflammation of the mucous membrane and skin areas. In 10 patients, many epithelial cells were found in smears, which indicated the intensity of the inflammation process. A large number of leukocytes (from 30 to 70 leukocytes per field of view) was found in 7 patients, a slightly smaller number (up to 30 per field of view) was found in the remaining 23 patients. Massive leukocytosis in the contents of the vagina, cervical and urethral canals is characteristic of infectious inflammation caused by bacteria and fungi.
All patients were treated with Immunomax for recurrent anogenital warts of the mucous membranes and (or) skin caused by the human papillomavirus. Treatment was carried out by using injections of Immunomax solution. The freeze-dried drug “Immunomax 200 units” was used. The drug was dissolved before use; the solutions were not stored. The contents of the bottle (200 units) were dissolved in 2 ml of sterile water for injection, and the resulting solution was administered intramuscularly. The procedure was repeated 6 times on days 1, 2, 3, 8, 9, 10 of therapy.
The course of treatment with Immunomax described above consisted of 6 intramuscular injections over 10 days (single dose of Immunomax 200 units, course dose 1200 units). This course of treatment was used in 20 of the 30 patients studied. In the remaining 10 patients, a single dose of 100 IU of Immunomax was used. In this case, 1 ml (100 IU) of Immunomax solution was used for intramuscular administration (the contents of the 200 IU bottle were dissolved in 2 ml of sterile water for injection, and 1 ml (100 IU), half of the resulting solution, was used for intramuscular administration). That is, the course of treatment in 10 patients consisted of 6 intramuscular injections over 10 days (single dose of Immunomax 100 units, course dose 600 units).
Research results and discussion
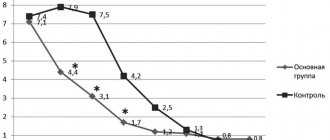
As can be seen from the results of Table 3, before treatment, 12 (40%) of the 30 studied had a large number of oncogenic types of human papillomavirus in the material from the lesions (+++ or ++). In 2 (7%) patients, the number of oncogenic types of human papillomavirus was moderate (+). As for non-oncogenic types of human papillomavirus, a large number of them were detected before treatment in 14 (47%) of 30 examined in the material from the lesions (+++ or ++). It should be noted that in 14 (47%) patients the number of non-oncogenic types of human papillomavirus was moderate (+).
Immediately after treatment with Immunomax, in 8 (27%) of the 30 subjects studied, a large number of oncogenic types of human papillomavirus were observed in the material from the lesions (+++ or ++). In 2 (7%) patients, the number of oncogenic types of human papillomavirus was moderate (+). Non-oncogenic types of human papillomavirus were detected in large quantities in 4 (13%) of 30 examined in the material from the lesions (+++ or ++), and in moderate quantities (+) were not detected in any patient.
3 months after treatment with Immunomax, only 3 (10%) of the 30 examined had a large number of oncogenic types of human papillomavirus in the material from the lesions (+++ or ++), in 1 (3%) patient the number of oncogenic types of papillomavirus person was moderate (+). Non-oncogenic types of human papillomavirus were detected in large quantities in 2 (7%) of 30 examined (+++ or ++), and in moderate quantities (+) were not detected in any patient.
Consequently, etiological cure was achieved in 26 (87%) patients. In two patients, during a control study 3 months after treatment, both oncogenic and non-oncogenic types of the virus were detected.
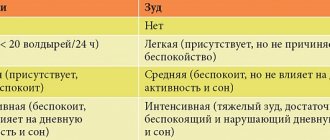
Subjective sensations (itching, burning) associated with the inflammatory process disappeared in all patients. In 6 out of 30 patients, minor discomfort remained (Table 4).
It is necessary to take into account the fact that doctors noted the disappearance of warts (condylomas) ranging in size from 0.1 to 0.3 cm within 2–4 days after the first injection of Immunomax. Changes in large warts (condylomas) were not monitored, since they were removed simultaneously with Immunomax therapy. 3 months after treatment with Immunomax, only 4 (13%) of 30 patients experienced the reappearance of anogenital warts. It should be especially noted that in all 4 patients there was a sharp decrease in the number of warts from 10–17 to 2–3 in each patient, and the remission lengthened by 2–3 times. Complete clinical and etiological cure was observed in 26 (87%) of 30 patients.
It should also be noted that monotherapy with Immunomax caused the disappearance of a number of concomitant infections: Herpes simplex virus
(type 2) - in 6 out of 7 patients,
Ureaplasma urealyticum
- in 5 out of 7 patients,
Mycoplasma hominis
- in 6 out of 6 patients,
Trichomonas vaginalis
- in 1 out of 1 patients,
Candida albicans
- in 2 out of 8.
conclusions
1. Intramuscular use of Immunomax solution (6 injections over 10 days, single dose 200 units, course dose 1200 units) is well tolerated by patients and does not cause any local or general toxic or allergic reactions.
2. The use of Immunomax turned out to be completely safe in people with a history of allergies.
3. Immunomax is an effective treatment for recurrent anogenital warts caused by the human papillomavirus. The use of Immunomax for the treatment of recurrent anogenital warts caused by the human papillomavirus, according to the results of this study, achieved clinical cure in 87%, etiological cure in 87% of patients.
4. For the treatment of recurrent anogenital warts caused by the human papillomavirus, Immunomax injections should be used (100-200 units intramuscularly, per course of treatment - 6 injections on days 1, 2, 3, 8, 9, 10 of treatment).
5. To treat an infection caused by the herpes simplex virus, Immunomax injections should be used (100-200 units intramuscularly, per course of treatment - 6 injections on days 1, 2, 3, 8, 9, 10 of treatment).
6. For the treatment of infections caused by chlamydia, mycoplasma, ureaplasma or trichomonas, Immunomax should be used (intramuscular 100–200 units, per course of treatment - 6 injections on days 1, 2, 3, 8, 9, 10 of treatment) in combination with antibiotics.
7. The effectiveness of Immunomax treatment for recurrent anogenital warts caused by the human papillomavirus should be determined 3 months after treatment based on the results of a control study for the human papillomavirus, as well as the absence of recurrent rashes of anogenital warts.
Literature:
1. “Post-translational modification of self-proteins leads to a looseness of tolerance” XI International Congress of Immunology, Stockholm. 22 – 27 Jul 2001. Doyle, Hester, Geerenelle, Mamula.
2. “Skin and venereal diseases.” Guide for doctors. Edited by Yu.K. Skripkina M 1995
3. “Hapillomavirus infection.” M.A. Bashmakova, A.M. Savicheva, 2001
4. “Viral, chlamydial and mycoplasma diseases of the genitals.” Guide for doctors. IN AND. Kozlova, A.F. Puchner. M 1997
5. “Advances in diagnosing STDs using the polymerase chain reaction method” S.N. Shcherbo, A.L. Tishchenko, 1999