Heptral®
Ademetionine belongs to the group of hepatoprotectors and also has antidepressant activity. It has choleretic and cholekinetic effects, has detoxification, regenerating, antioxidant, antifibrosing and neuroprotective properties.
Replenishes the deficiency of S-adenosyl-L-methionine (ademetionine) and stimulates its production in the body; it is found in all environments of the body. The highest concentration of ademetionine was observed in the liver and brain. Plays a key role in the metabolic processes of the body, takes part in important biochemical reactions: transmethylation, transsulfurization, transamination. In transmethylation reactions, ademetionine donates a methyl group for the synthesis of cell membrane phospholipids, neurotransmitters, nucleic acids, proteins, hormones, etc. In transsulfuration reactions, ademetionine is a precursor of cysteine, taurine, glutathione (providing a redox mechanism of cellular detoxification), coenzyme A (included in biochemical reactions of the tricarboxylic acid cycle and replenishes the energy potential of the cell). Increases the content of glutamine in the liver, cysteine and taurine in plasma; reduces the content of methionine in serum, normalizing metabolic reactions in the liver. After decarboxylation, it participates in aminopropylation reactions as a precursor of the polyamines putrescine (stimulator of cell regeneration and hepatocyte proliferation), spermidine and spermine, which are part of the ribosome structure, which reduces the risk of fibrosis. Has a choleretic effect. Ademetionine normalizes the synthesis of endogenous phosphatidylcholine in hepatocytes, which increases membrane fluidity and polarization. This improves the function of bile acid transport systems associated with hepatocyte membranes and promotes the passage of bile acids into the biliary tract. Effective for intralobular cholestasis (impaired synthesis and flow of bile). Ademetionine reduces the toxicity of bile acids in hepatocytes by conjugating and sulfating them. Conjugation with taurine increases the solubility of bile acids and their removal from the hepatocyte. The process of sulfation of bile acids facilitates their elimination by the kidneys, facilitates their passage through the hepatocyte membrane and excretion in the bile. In addition, sulfated bile acids themselves additionally protect liver cell membranes from the toxic effects of non-sulfated bile acids (present in high concentrations in hepatocytes during intrahepatic cholestasis). In patients with diffuse liver diseases (cirrhosis, hepatitis) with intrahepatic cholestasis syndrome, ademetionine reduces the severity of skin itching and changes in biochemical parameters, incl. concentration of direct bilirubin, alkaline phosphatase activity, aminotransferases, etc. The choleretic and hepatoprotective effect lasts up to 3 months after cessation of treatment. It has been shown to be effective against hepatopathy caused by various hepatotoxic drugs. Prescription to patients with opioid addiction accompanied by liver damage leads to regression of clinical manifestations of withdrawal, improvement of the functional state of the liver and microsomal oxidation processes. Antidepressant activity appears gradually, starting from the end of the first week of treatment, and stabilizes within 2 weeks of treatment. Effective for recurrent endogenous and neurotic depressions resistant to amitriptyline. Has the ability to interrupt relapses of depression. Ademetionine increases the synthesis of proteoglycans and leads to partial regeneration of cartilage tissue.
Heptral powder lyophilisate with solvent ampoules 400 mg 5 pcs
Ademetionine belongs to the group of hepatoprotectors. It has a choleretic effect, has detoxifying, regenerating, antioxidant, antifibrosing and neuroprotective properties.
Ademetionine (S-adenosyl-L-methionine) is a naturally occurring amino acid that is present in virtually all tissues and body fluids. The highest concentration of ademetionine was observed in the liver and brain. Ademetionine is a derivative of the sulfur-containing amino acid methionine. Plays a key role in the body's metabolic processes and takes part in important biochemical reactions. Participates in the synthesis of phospholipids of cell membranes, neurotransmitters, nucleic acids, proteins, hormones, etc. Ademetionine is a precursor of cysteine, taurine, glutathione (providing a redox mechanism of cellular detoxification), coenzyme A (included in the biochemical reactions of the tricarboxylic acid cycle and replenishes energy potential cells). Increases the content of glutamine in the liver, cysteine and taurine in plasma; reduces the content of methionine in serum, normalizing metabolic reactions in the liver. Indirectly participates in stimulating the regeneration and proliferation of liver cells, which reduces the risk of fibrosis. Ademetionine normalizes the synthesis of endogenous phosphatidylcholine in hepatocytes, which increases membrane fluidity and polarization. This improves the function of bile acid transport systems associated with hepatocyte membranes and promotes the passage of bile acids into the biliary tract. Effective for intralobular cholestasis (impaired synthesis and flow of bile). Ademetionine reduces the toxicity of bile acids in liver cells. In patients with diffuse liver diseases (cirrhosis, hepatitis), ademetionine reduces the severity of skin itching and helps improve biochemical blood parameters, incl. concentration of direct bilirubin, alkaline phosphatase activity, aminotransferases, etc. The choleretic and hepatoprotective effect lasts up to 3 months after cessation of treatment. It has been shown to be effective against hepatopathy caused by various hepatotoxic drugs. A number of studies have confirmed the effectiveness of ademetionine in the treatment of fatigue in patients with chronic liver diseases.
Heptral
Hepatoprotector, has antidepressant activity. It has choleretic and cholekinetic effects. It has detoxifying, regenerating, antioxidant, antifibrosing and neuroprotective properties.
Replenishes ademetionine deficiency and stimulates its production in the body; it is found in all environments of the body. The highest concentration of ademetionine was observed in the liver and brain. Plays a key role in the metabolic processes of the body, takes part in important biochemical reactions: transmethylation, transsulfurization, transamination. In transmethylation reactions, ademetionine donates a methyl group for the synthesis of cell membrane phospholipids, neurotransmitters, nucleic acids, proteins, hormones, etc. In transsulfation reactions, ademetionine is a precursor of cysteine, taurine, glutathione (providing a redox mechanism for cellular detoxification), acetylation coenzyme (included in biochemical reactions of the tricarboxylic acid cycle and replenishes the energy potential of the cell).
Increases the content of glutamine in the liver, cysteine and taurine in plasma; reduces the content of methionine in serum, normalizing metabolic reactions in the liver. After decarboxylation, it participates in the processes of aminopropylation as a precursor of polyamines - putrescine (stimulator of cell regeneration and proliferation of hepatocytes), spermidine and spermine, which are part of the structure of ribosomes, which reduces the risk of fibrosis.
Has a choleretic effect. Ademetionine normalizes the synthesis of endogenous phosphatidylcholine in hepatocytes, which increases membrane fluidity and polarization. This improves the function of bile acid transport systems associated with hepatocyte membranes and promotes the passage of bile acids into the biliary system. Effective for intrahepatic (intralobular and interlobular) variants of cholestasis (impaired synthesis and flow of bile). Ademetionine reduces the toxicity of bile acids in hepatocytes by conjugating and sulfating them. Conjugation with taurine increases the solubility of bile acids and their removal from the hepatocyte. The process of sulfation of bile acids facilitates their elimination by the kidneys, facilitates their passage through the hepatocyte membrane and excretion in the bile. In addition, sulfated bile acids themselves additionally protect liver cell membranes from the toxic effects of non-sulfated bile acids (present in high concentrations in hepatocytes during intrahepatic cholestasis). In patients with diffuse liver diseases (cirrhosis, hepatitis) with intrahepatic cholestasis syndrome, ademetionine reduces the severity of skin itching and changes in biochemical parameters, incl. level of direct bilirubin, alkaline phosphatase activity, aminotransferases. The choleretic and hepatoprotective effect lasts up to 3 months after cessation of treatment.
Shown to be effective in hepatopathies caused by hepatotoxic drugs.
Prescription to patients with opioid addiction accompanied by liver damage leads to regression of clinical manifestations of withdrawal, improvement of the functional state of the liver and microsomal oxidation processes.
Antidepressant activity appears gradually, starting from the end of the first week of treatment, and stabilizes within 2 weeks of treatment. The drug is effective for recurrent endogenous and neurotic depression resistant to amitriptyline. Has the ability to interrupt relapses of depression.
Prescribing the drug for osteoarthritis reduces the severity of pain, increases the synthesis of proteoglycans and leads to partial regeneration of cartilage tissue.
Pharmacokinetics
The tablets are film-coated, dissolving only in the intestines, due to which ademetionine is released in the duodenum.
Suction
The bioavailability of the drug when taken orally is 5%, increases when taken on an empty stomach. Cmax of ademetionine in plasma is dose-dependent and is 0.5-1 ml/l 3-5 hours after a single oral dose in doses of 400 to 1000 mg. Cmax of ademetionine in plasma decreases to the initial level within 24 hours.
Distribution
Binding to plasma proteins is insignificant, ≤ 5%. Penetrates through the BBB. There is a significant increase in the concentration of ademetionine in the cerebrospinal fluid.
Metabolism
Biotransformed in the liver. The process of formation, consumption and re-formation of ademetionine is called the ademetionine cycle. In the first step of this cycle, ademetionine-dependent methylases use ademetionine as a substrate to produce S-adenosylhomocysteine, which is then hydrolyzed to homocysteine and adenosine by S-adenosylhomocysteine hydrolase. Homocysteine, in turn, undergoes reverse transformation to methionine by transfer of a methyl group from 5-methyltetrahydrofolate. Eventually, methionine can be converted to ademetionine, completing the cycle.
Removal
T1/2 - 1.5 hours. Excreted by the kidneys. In studies in healthy volunteers, ingestion of labeled (methyl 14C) S-adenosyl-L-methionine in urine showed 15.5 ± 1.5% radioactivity after 48 hours, and in feces - 23.5 ± 3.5% radioactivity after 72 hours. Thus, about 60% was deposited.
Psychiatry Psychiatry and psychopharmacotherapy named after. P.B. Gannushkina No. 06 2000
Synonyms: Ademetionine(rINN). Ademethionine: S-Adenosyl-L-meothine(SAMe): Methioninyl adenylate; (S)-5′-[(3-amino-carboxypropyl)methylsulphonio]-5'deoxyadenosine hydroxyde, inner salt; Gross formula and molwes C 15 H 22 N 6 O 5 S = 398.4
Introduction
Biological methylation processes play a key role in the etiology of mental disorders.
The main source and effective donor of methyl groups in the central and peripheral nervous system is ademetionine (heptral) - an active sulfur-containing metabolite of methionine, a natural antioxidant and antidepressant, formed in the liver in amounts up to 8 g / day and present in all tissues and fluids of the body, and most of all - in places of education and consumption, i.e. in the liver and brain. In this regard, two particularly important biochemical processes in the liver are the synthesis of methionine and S-adenosyl-L-methionine through homocysteine methylation. The enzymes S-adenosyl-methionine synthetase and methionine adenosyl transferase (MAT) are involved in the formation and functioning of ademetionine. The latter is encoded on two genes - MAT1A and MAT2A, which catalyze the formation of SAM, and the expression of only MAT2A is associated with faster cell proliferation. The circular DNA of human SAM synthetase includes 3217 nucleotides encoding a protein of 395 amino acid residues with a molecular weight of 43,647 daltons. In the messenger RNA of the human genome, a single region is responsible for encoding this protein, and the structural features of the liver-specific S-adenosylmethionine synthetase gene in humans and rats turned out to be quite similar. The idea of using SAM as an independent drug is based on qualitative and quantitative correlations of the severity of mental and somatic disease states in humans and animals with doses and content of SAM in normal or pathologically altered target tissues of the body. The purpose of this information and analytical review is more pragmatic and is to summarize and compare the observed clinical effectiveness and safety of Heptral with existing drugs prescribed for the same indications. The most important and recognized of these indications are exo- and endogenous depression, alcohol withdrawal, liver pathologies and arthralgia. Sources of information 1. Medical and biological data from the Internet (Medline, Pubmed, etc.).
2. Thematic abstract journals of VINITI RAS. 3. Systematic reviews of the Cochrane Library (Oxford, UK) for 1984-2000. Heptral is an antidepressant
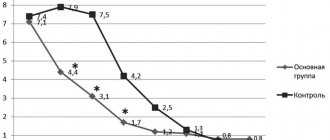
The relative harmlessness of heptral made it possible to evaluate its effect on the vital signs of healthy people. In particular, the heart rate and concentration of adrenaline in the plasma of healthy subjects decreased moderately with daily administration of 400 mg of heptral for a week in the same way as under the influence of MAO inhibitors, but the plasma level of MNPG did not depend on the intake of heptral. An increase in adrenaline levels when changing body position from horizontal to vertical was easily stopped by taking heptral, which indirectly confirms the presence of antidepressant properties. A statistical synthesis (meta-analysis) of the results of 19 comparative clinical trials involving 498 patients with depression of varying severity made it possible to establish a significant, 38-60%, excess of the antidepressant activity of heptral over the activity of placebo and the coincidence of the intensity of its action with the antidepressant effects of standard tri- and heterocyclic drugs - imipramine, desipramine, amitriptyline and others with an almost complete absence of their inherent side effects. In standard clinical trials, Heptral was statistically significantly more effective than placebo and tricyclic antidepressants in recurrent endogenous and neurotic depression resistant to amitriptyline, differing from them in its ability to interrupt relapses and the absence of side effects. Almost all researchers note a more rapid development and stabilization of the antidepressant effect of heptral (1 and 2 weeks, respectively) compared to standard drugs, especially when administered parenterally. In particular, in an open multicenter clinical study of 195 depressed patients, remission occurred after 7-15 days of parenteral administration of heptral 400 mg/day, and when it was combined with tricyclic antidepressants, the effects were significantly faster and more pronounced than when they were combined with placebo. It should be noted that with exacerbation of depressive symptoms, the level of ademetionine in the blood and tissues decreases, which requires increasing dosages.
Heptral for withdrawal symptoms
The experience of testing and using heptral for alcohol withdrawal and opium addiction is apparently quite limited, since there are no English-language publications on this topic in available Internet databases.
The following are the main results of two domestic clinical trials of heptral, conducted in the department of clinical psychopharmacology of the Research Institute of Narcology of the Ministry of Health of the Russian Federation (Director of the Research Institute, Corresponding Member of the Russian Academy of Medical Sciences, Prof. N.N. Ivanets) and at the Central Research Institute of Gastroenterology and the 17th Narcological Hospital of Moscow . 1. Heptral for alcohol withdrawal
Stopping habitual alcohol consumption is fraught with fatal complications.
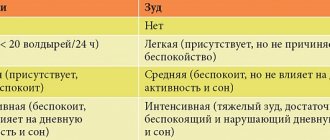
Clinically, alcohol withdrawal is manifested by tremors, hallucinations, seizures and delirium, infectious and somatic diseases and injuries. Symptoms appear after a few hours and gradually disappear over 2-3 days. Alcoholic delirium usually develops in 5-10% of cases after 3-4 days and also includes trembling, agitation, confusion, loss of orientation and a sharp increase in autonomic activity - fever, tachycardia and profuse sweating; mortality rate is about 5%. Seizures are quite rare, occur 12-48 hours after alcohol withdrawal and are usually generalized; their number is small and they can be treated with conventional drugs. The purpose of the study was to study the hepatotropic effect and effectiveness of Heptral in the treatment of depressive disorders and pathological craving for alcohol (with alcohol withdrawal syndrome) in an open study. We examined 20 alcoholic men aged 30-60 years with a disease duration of 6-25 years, 12 of them with hereditary complications. All patients were diagnosed with stage II alcoholism: with a predominance of the pseudo-binge form - in 14, a permanent form against the background of high tolerance - in 6 patients. All of them had diagnostic criteria for stage II alcoholism: primary pathological craving for alcohol, a pronounced symptom of “loss of control”, maximum tolerance to alcohol, fully formed alcohol withdrawal syndrome (AAS) - morning hangover, altered patterns of intoxication in combination with amnesia of the period of intoxication, exacerbation premorbid character traits, adverse social and somatic consequences of the disease. AAS was accompanied by the usual somatovegetative and psychopathological disorders. The severity of AAS was assessed as mild in 6 and moderate in 14 patients, with a Hamilton scale score of at least 14 points, which was a criterion for inclusion in the study along with pathological craving for alcohol and liver pathology. All patients had liver enlargement, 17 were diagnosed with alcoholic fatty hepatosis, and 3 were diagnosed with chronic alcoholic hepatitis. Heptral was prescribed 2 bottles parenterally (800 mg) for 2 weeks. and then 1 tablet (200 mg) 4 times a day for the next 2 weeks. Along with heptral, vitamins of groups B and C were prescribed and, if necessary, antihypertensive drugs (magnesium sulfate) and benzodiazepines (only at night) in the first 2 days. Results.
The therapeutic effect was noted on days 2-4 of treatment.
Fear and anxiety disappeared, irritability decreased, asthenia decreased and physical condition improved, blood pressure normalized, appetite appeared, tremor and hyperhidrosis disappeared. By the end of the week, mood leveled out, sleep was restored, depressive symptoms decreased by 60-70%, and after 4 weeks, depressive disorders were completely relieved. The craving for alcohol decreased on average by the 10th day. According to the general impression scale, the results were assessed as a significant improvement in depression and as moderate in terms of craving for alcohol and hepatotropic effects (a tendency towards positive dynamics of liver function and a decrease in its size). The drug was well tolerated and there were no side effects, complications or addiction to it. In the instructions for the use of heptral, it is recommended to prescribe it for intrahepatic cholestasis induced by liver lesions of various origins, cirrhotic and pre-cirrhotic conditions, encephalopathies of secondary origin, depressive syndromes (including secondary) and withdrawal syndrome. Heptral is contraindicated in case of individual hypersensitivity to it, in the first two trimesters of pregnancy and lactation. In cirrhotic and precirrhotic conditions associated with hyperazotemia, oral administration of Heptral should be carried out under medical supervision and monitoring of nitrogen levels. Heptral is not recommended for use in children without strict indications. No interactions of Heptral with other drugs were observed and no clinical cases of overdose were noted. 2. Heptral for opium withdrawal.
Under observation were 20 sick men 17-38 years old with a diagnosis of “2nd degree opium addiction, withdrawal syndrome” and with a disease duration of 1-22 years; in 50% of cases, the course of drug addiction was aggravated by taking diphenhydramine (1-2 tablets per drug injection). Treatment consisted of heptral administered intravenously at 800 or 1600 mg/day in the first 14 days and 1600 mg/day in tablets for the next 14 days. An improvement in the functional state of the liver and stimulation of microsomal oxidation processes were observed, which resulted in increased clearance and accelerated elimination of the marker drug antipyrine. A reverse development of clinical manifestations of withdrawal and a clear antidepressant effect were also noted.
Heptral - hepatoprotector
Most etiological factors of intrahepatic cholestasis lead to inhibition of the activity of S-adenosylmethyl synthetase and a decrease in the production of S-ademetionine, which is accompanied by disruption of biochemical processes in hepatocytes - transmethylation and transsulfidation.
As a result, the following decreases: the content of phospholipids, the activity of Na+K+-ATPase and other carrier proteins, membrane fluidity, the uptake and excretion of bile components, cellular reserves of thiols and sulfates (glutathione, taurine, etc.), which have a pronounced antioxidant effect and are the main substances in the detoxification of endo- and exogenous xenobiotics. The deficiency of these products leads to cytolysis of hepatocytes in cholestasis of any origin. Clinical manifestations of cholestasis
are quite similar, these are:
1. excessive flow of bile elements into the blood;
2. reduction in the amount or absence of bile in the intestines; 3. the effect of bile components on liver cells and tubules. Regurgitation of bile into the blood induces skin itching, jaundice, xanthomas, xanthelasmas, dark urine and systemic lesions:
- acute renal failure;
- development of ulcers, erosions and bleeding in the stomach;
- increased risk of endotoxemia and septic complications.
At the same time, a deficiency of bile in the intestines is fraught with steatorrhea and malabsorption syndrome, a deficiency of fat-soluble vitamins, and impaired bone mineralization. Excessive amounts of bile components lead to necrosis of hepatocytes and tubules and to hepatic cellular failure, and with prolonged cholestasis, cirrhosis is formed with the development of ascites, edema and hepatic encephalopathy. Often, cholestasis (for example, drug-induced) is asymptomatic and its only manifestation is the results of biochemical liver tests. The etiological effect on cholestasis is problematic and most patients are prescribed pathogenetic and symptomatic treatment. Heptral is the drug of choice in most cases for the following mechanisms and causes of cholestasis:
- Reduced fluidity (permeability) of the basolateral and/or canalicular membrane of hepatocytes during pregnancy, alcoholic and drug-induced liver damage.
- Inhibition of Na+K+-ATPase and other membrane transport proteins in drug and/or bacterial liver damage.
- Destruction of the cytoskeleton of hepatocytes, disruption of vesicular transport in viral, alcoholic and drug-induced hepatitis, cirrhosis, endotoxemia, sepsis, benign recurrent cholestasis.
- Violation of the integrity of the tubules (membranes, microfilaments, cellular junctions) under the influence of drugs, oral contraceptives, bacterial infections, Bailer's disease.
Clinical trials:
- In an open clinical trial, treatment with Heptral for alcoholic liver diseases (34 people) for 14 days intravenously at a dose of 800 mg/day, and for the next 14 days at a dose of 400 mg/day 2 times orally (tablets) led to the disappearance of signs of depression, improvement of biochemical and physical (liver density) indicators.
- Treatment of 23 patients with chronic hepatitis C with interferon a2 was accompanied by the development of cholestatic and depressive syndromes. Signs of cholestasis appeared in the first 3 months of treatment and were especially pronounced in patients with cirrhotic transformation of the liver. The inclusion of heptral in the complex of therapy helped to promptly stop depressive and cholestatic symptoms and carry out a full course of antiviral therapy with interferon a2.
- During the treatment of 8 patients with drug-induced liver damage, an improvement in general condition and normalization of liver tests was observed.
- Heptral was prescribed to 32 patients with chronic diffuse liver diseases and intrahepatic cholestasis, 16 of whom had primary biliary cirrhosis. During the first 16 days of phase I of treatment, heptral was administered intravenously at a dose of 800 mg/day, and in the next 16 days at a dose of 1600 mg/day. In most patients, a pronounced positive effect was observed - the symptoms of asthenia, skin itching, jaundice disappeared, as well as a statistically significant normalization of biochemical parameters. In patients with primary biliary cirrhosis, there was a tendency to reduce cholesterol and bilirubin in the blood. With repeated courses, satisfactory tolerability of heptral and absence of resistance to its positive effects were noted.
Heptral is an analgesic
Osteoarthritis is a degenerative joint disease characterized by progressive catabolic loss (“wear and tear”) of articular cartilage due to an imbalance between the synthesis and degradation of cartilage proteoglycans, with subsequent bone growths at the edges of the articular surfaces. It mainly develops in older people, but can occur at any age, especially due to injury, chronic inflammatory diseases and congenital joint defects. Most often, the distal and proximal interphalangeal joints of the hands, hip and knee joints, and the cervical and lumbar spine are affected. Spondyloarthrosis sometimes leads to a narrowing of the spinal canal (caudogenic intermittent claudication), pain in the legs and buttocks when standing or walking. Accompanied by severe bone and muscle pain. The goal of therapy is to relieve pain and prevent disability. Nonsteroidal anti-inflammatory drugs (NSAIDs) may provide short-term pain relief, but long-term use can cause harmful side effects (such as stomach bleeding) and increased cartilage loss. Empirical use of Heptral in osteoarthritis resulted in pain relief comparable to NSAIDs with a complete absence of side effects, as well as stimulation of proteoglycan synthesis and partial regeneration of cartilage tissue.
Conclusion
The potential of heptral, alone and in combination with other drugs, in the treatment of depressive disorders is far from exhausted. As already noted, in the available Internet databases there are no English-language publications on the treatment of withdrawal syndrome with Heptral, and in a random sample of 18 domestic articles, only non-steroidal anti-inflammatory drugs were used in the treatment of arthralgia. Further study of the effectiveness of heptral in the treatment of affective disorders, inhibition of prolactin secretion, etc. remains to be done. Pharmacokinetics have not been studied at all, although the relationship between the antidepressant effectiveness of ademetionine and its concentration in the blood and tissues appears explicitly or implicitly in many studies, which suggests the possibility of searching for optimal treatment regimens among the existing ones . So far, it has been found that its transport through the villi of human placenta preparations occurred rather slowly, similar to the passive diffusion of L-glucose, and was accompanied by a non-enzymatic transformation to a metabolite of an unknown structure. The transport of melatonin and its marker drug antipyrine proceeded most quickly, while vitamin E diffused 10 times slower than L-glucose and ademetionine, but its non-racemic free forms diffused significantly faster. Measurable concentrations of ademetionine after parenteral administration were found in the CSF of patients with senile dementia, which gives hope for new opportunities in the treatment of this and other neurodegenerative diseases with heptral.