Despite obvious progress in the cultural and social spheres of man, parasitic skin diseases are still a pressing problem in dermatology. Most often, doctors have to deal with diseases caused by ticks. Parasites for the host are foreign organisms that secrete various substances, in response to which corresponding reactions develop: cellular (proliferation and hypertrophy), tissue (inflammation - vascular reaction, exudation, edema, infiltration), as well as systemic humoral (immune) changes. Parasitic skin diseases are divided into superficial (localized on the surface and inside the epidermis) and deep (dermis, subcutaneous fat). The most common parasitic skin diseases are scabies and demodicosis.
Demodicosis is a common pathology in humans and animals caused by mites of the genus Demodex.
In the general structure of skin diseases, demodicosis accounts for 2.9%, and in the structure of acneiform dermatoses - 10.5%.
Currently, of the 65 species and several subspecies of Demodex, only two are found in humans: Demodex folliculorum and Demodex brevis.
Each species and subspecies of demodex is strictly specific to its owner.
The iron mite (Demodex folliculorum)
is the most common, found only in humans in hair follicles and sebaceous glands; outside the host, its reproduction stops [7]. The tick is viable outside the host at constant humidity and room temperature in the dark for up to 9 days. The optimal temperature for tick development is 30–40 °C; at a temperature of 14 °C the ticks are in a state of torpor, and at 52 °C they quickly die. Insects can live up to 25 days in water; in dry air they die after 1.5 days. The most favorable nutrient medium for demodex is vegetable oil, fat, and petroleum jelly. The pincers measure 0.3–0.4 mm. In the cavity of the hair follicle, females lay eggs, from which after 60 hours a larva hatches, which is motionless and constantly feeds. After 40 hours, the larva turns into nymph 1, which is also inactive and remains in the follicle. After 72 hours, it transforms into nymph 2, mobile, moving along the skin, and after 60 hours, it transforms into an adult. The adult re-enters the follicle and dies after laying eggs. The life cycle of a tick is about 15 days. [4].
If there are any pathological processes in the host's body - neuroendocrine, gastrointestinal, mental, immune, as well as in the presence of foci of chronic infection, the body becomes sensitized to the mite. In this situation, demodex is a chemical, mechanical irritant that contributes to the development and maintenance of the pathological process. In addition, the symbiosis with corynebacteria and opportunistic flora is disrupted, which is also a trigger factor for the development of the disease. It is also possible to carry asymptomatic ticks in the absence of skin pathology. The greatest activity of demodex on human skin is observed in the spring-autumn period, which is associated with increased insolation, changes in ambient temperature, immune and endocrine changes. Most often, the tick is found in the area of the nasolabial fold, cheeks, nose, chin, quite rarely in the neck area and very rarely in the back and chest area.
Clinical manifestations of demodicosis are varied. There are skin and ocular manifestations of the disease. It is necessary to distinguish between demodicosis itself and diseases the course of which is aggravated by the presence of mites. The most common diseases are presented in table. 1.
For the choice of therapy, the clinical picture of the disease, the presence of forms of mites, and their number are important. It is also necessary to take into account the patient's concomitant pathology. To act directly on demodex mites, acaricidal agents are used,
which include derivatives of the nitroimidazole group.
Until recently, metronidazole, used for 4 to 6 weeks, was considered the most effective drug from this group. [3, 8, 9]. There is no consensus on the mechanism of action of the drug. It is still not clear which of its pharmacodynamic properties plays a leading role in achieving a clinical effect in the treatment of demodicosis. It has been established that metronidazole enhances the protective and regenerative functions of the mucous membrane of the stomach and intestines [1, 2] and has a pronounced anti-edematous effect [14]. The drug has a bacteriostatic effect against gram-negative anaerobic bacilli [16], as well as an antiparasitic effect against Demodex folliculorum
[1]. Experimental and clinical studies have shown a suppressive effect of the drug on some indicators of cellular immunity, and suppression of leukocyte chemotaxis has also been noted [13, 15].
In general, the drug is well tolerated, but side effects are possible during therapy: headache, nausea, vomiting, dry mouth, urticaria, itching, leukopenia, candidiasis. In addition, in recent years, cases of failure in the treatment of demodicosis with metronidazole have become much more frequent. It is possible that treatment failures may be associated with the emergence of resistance of bacterial-parasitic flora to metronidazole, which has been used for more than 40 years.
Recently, there have been reports of the effective treatment of rosacea and demodicosis with a relatively new nitroimidazole derivative, ornidazole: a-(chlorome-thyl)-2-methyl-5-nitroimidazole-1-ethanol [12]. Not only the antiparasitic effect of the drug was noted, but also bacteriostatic, increasing the activity of neutrophils, stimulating adrenergic structures, and enhancing reparative processes [2].
Goal of the work:
determination of the effectiveness of the use of ornidazole in the complex treatment of demodicosis.
Pharmacological properties of the drug Ornidazole
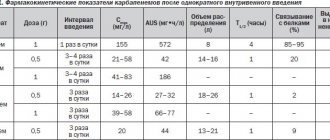
Ornidazole is an imidazole derivative that is active against Trichomonas vaginalis , Entamoeba histolytica , Giardia lamblia ( Giardia intestinalis ), as well as some anaerobic bacteria such as Bacteroides and Clostridium spp ., Fusobacterium spp ., and anaerobic cocci. After oral administration, 90% of ornidazole is rapidly absorbed. The maximum concentration in blood plasma is achieved within 3 hours. The binding of ornidazole to plasma proteins is about 13%. Depending on the dosage regimen, the optimal concentration of the active substance in the blood plasma ranges from 6 to 36 mcg/l. Penetrates very well into CSF, other biological fluids and body tissues. The half-life is approximately 13 hours. After a single dose of ornidazole, 85% of the dose is eliminated within the first 5 days. Excretion occurs mainly in urine (63%) and feces (22%). About 4% of the dose taken is excreted unchanged by the kidneys.
Use of the drug Ornidazole
Inside, after eating. For trichomoniasis, 500 mg is prescribed 2 times a day (morning and evening) for 5 days. To eliminate the possibility of re-infection, the sexual partner must undergo the same course of treatment. The daily dose for children is 25 mg/kg in 1 dose. For amebiasis, the following treatment regimens are possible: a 3-day course of treatment for patients with amoebic dysentery and a 5-10-day course of treatment for all forms of amebiasis.
Duration of treatment, days | Daily dose | |
Adults and children weighing more than 35 kg | Children weighing up to 35 kg | |
| 3 | 1.5 g once in the evening. For body weight more than 60 kg - 2 g in equal doses in 2 doses (morning and evening) | 40 mg/kg once |
| 5–10 | 1 g in equal doses in 2 doses (morning and evening) | 25 mg/kg once |
For giardiasis, adults and children weighing more than 35 kg are prescribed 1.5 g once in the evening. Children weighing less than 35 kg - 40 mg/kg once. The duration of treatment is 1–2 days. To prevent infections caused by anaerobic bacteria - 1 g 1-2 hours before surgery; after surgery - 500 mg 2 times a day for 3–5 days. It is administered intravenously to adults and children over 12 years of age in a single dose of 0.5–1 g with an interval of 8–12 hours. The daily dose should not exceed 4 g. The course of treatment averages 5–10 days.
results
The main subjective manifestations of bacterial vaginosis in the examined women were pathological discharge of a creamy homogeneous nature from the genital tract, which was observed in 30 (100.0%) patients of the 1st group and in 29 (96.7%) patients of the 2nd group. Also, patients of the 1st and 2nd groups complained of an unpleasant “fishy” smell of discharge from the genital tract - 28 (93.3%) and 27 (90.0%), respectively, discomfort in the genital area - 16 (53, 3%) and 14 (46.7%) patients, respectively, and pain during sexual intercourse - 10 (33.3%) and 11 (36.7%) patients, respectively.
In all examined patients, microscopic examination of the vaginal contents revealed “key cells.” A positive amino test result was determined in 29 (96.7%) patients of the 1st group and in 30 (100%) of the 2nd group. Also, most patients showed an increased pH value of the vaginal contents - more than 4.5. Both groups were comparable in terms of clinical signs of bacterial vaginosis (Fig. 1).
Rice. 1. Results of clinical examination of patients before prescribing therapy (p>0.05).
The average severity of pathological discharge according to patients of the 1st group was 2.4, according to patients of the 2nd group - 2.2; unpleasant odor of discharge from the genital tract - 1.7 and 1.7; discomfort in the genital area - 1.2 and 0.8; pain during sexual intercourse – 0.6 and 0.6, respectively. According to the results of an objective examination, the average severity of pathological discharge in patients of the 1st group was 2.3, in patients of the 2nd group - 2.2.
When analyzing the qualitative composition of vaginal microflora, the following results were obtained :
A. vaginae
was identified in 12 (40.0%) patients of group 1 and 16 (53.3%) of group 2
, G. vaginalis
- in 28 (93.3%) and 29 (96.7%) patients, respectively.
Other microorganisms associated with bacterial vaginosis were also identified: Mobiluncus , Leptotrix
(Fig. 2).
Rice.
2. Results of laboratory tests of patients before prescribing therapy. According to the bacteriological study, the majority of the examined patients (83.3% of patients of the 1st group and 80.0% of the 2nd group) had representatives of the Lactobacillaceae
were not determined, in 3 (10%) and 3 (10%) patients of the study groups, respectively, the number of lactobacilli was at the level of 102-103 CFU/ml, and in 2 (6.7%) and 3 (10%) patients, respectively - at the level of 104-105 CFU/ml (Fig. 3).
Rice.
3. Results of assessing the lactobacillary component of the vaginal microflora before prescribing therapy in patients of the examined groups. After establishing that the clinical and laboratory data of the examined patients met the criteria for inclusion in the study, all patients were prescribed therapy according to the regimens given in the “Material and Methods” section.
14 days after the end of therapy, subjective and objective clinical indicators were assessed, and laboratory tests were performed.
According to the results of a survey of patients, it was found that 1 (3.3%) patient of the 1st group and 5 (16.6%) patients of the 2nd group complained of discharge from the genital tract. Also, 1 (3.3%) patient of the 1st group and 4 (13.3%) patients of the 2nd group complained of an unpleasant “fishy” smell of pathological discharge. Discomfort in the genital area bothered 4 (13.3%) patients of the 2nd group, pain during sexual intercourse was noted by 1 (3.3%) patient of the 2nd group.
When assessing the nature of vaginal discharge, the following data were obtained: in 1 (3.3%) patient of the 1st group and 4 (13.3%) of the 2nd group, the vaginal discharge had a creamy character and an unpleasant “fishy” odor, characteristic of a bacterial vaginosis; in 1 (3.3%) patient of group 2, vaginal discharge was cheesy and accompanied by hyperemia and swelling of the vaginal mucosa. A positive amino test result was determined in 4 (13.3%) patients of the 2nd group, a vaginal pH value of more than 4.5 - in 1 (3.3%) patient of the 1st group and 3 (13.3%) of the 2nd group. th group. Microscopic examination of the vaginal contents in 1 (3.3%) patient of the 1st group and 4 (13.3%) of the 2nd group revealed “key” cells, which also confirmed the diagnosis of bacterial vaginosis in the examined women (Fig. 4 ).
Rice. 4. Results of clinical examination of patients after therapy (p<0.05).
The severity of pathological discharge according to the patient of the 1st group was 2.0, according to the patients of the 2nd group - 2.2; unpleasant odor of discharge from the genital tract - 1.0 and 1.6, respectively; discomfort in the genital area - 0 and 1.6, respectively; pain during sexual intercourse - 0 and 1.0, respectively. According to the results of an objective examination, the severity of pathological discharge in the patient of the 1st group was 2.0, in the patients of the 2nd group - 2.2.
A qualitative assessment of the vaginal microflora revealed that
A. vaginae
and
Mobiluncus
were identified in the vaginal contents only in patients of group 2 in 3 (10.0%) and 2 (6.7%) subjects, respectively.
G. _ vaginalis
were detected in 1 (3.3%) patient of group 1 and 2 (6.7%) patients of group 2 (Fig. 5).
Rice.
5. Results of bacterioscopic examination of vaginal contents in patients of the examined groups after therapy (p<0.05). When assessing the lactobacillary component of the vaginal microflora after therapy, it was found that normalization of indicators (the number of lactobacilli at the level of 105-107 CFU/ml) was achieved in 21 (70.0%) patients of group 1 and in 14 (46.7% ) patients of group 2. It was noteworthy that 10 (33.3%) patients of group 2 had representatives of the Lactobacillaceae
were not determined; in group 1 this figure was 10% (Fig. 6).
Rice.
6. Results of assessment of the lactobacillary component of the vaginal microflora in patients of the examined groups after therapy (p<0.05). There was no significant difference in the ease of use of the drugs. However, when analyzing adverse drug events, it was revealed that 6 (20.0%) patients of group 2 experienced side effects from the gastrointestinal tract (nausea, metallic taste in the mouth, pain in the epigastric region, diarrhea) during treatment with metronidazole ). In 1 (3.3%) patient of group 1, discomfort in the epigastric region was recorded during treatment, which resolved spontaneously after completion of therapy.
When monitoring vital signs, no clinically significant deviations were identified in any of the examined patients.
Special instructions for the use of the drug Ornidazole
Caution should be exercised when using ornidazole in patients with diseases of the central nervous system, such as epilepsy, brain lesions, and multiple sclerosis. If recommended doses are exceeded, the risk of side effects increases in children, patients with liver damage, alcohol abusers, and pregnant and breastfeeding women. Animal studies have not revealed any teratogenic or toxic effects of ornidazole on the fetus. No controlled clinical studies have been conducted in pregnant women; however, ornidazole can be prescribed in early pregnancy or during breastfeeding only for absolute indications.
Discussion
According to the data obtained, clinical signs of bacterial vaginosis after treatment were significantly more often recorded in patients of group 2 compared with those in patients of group 1: in 4 (13.3%) and 1 (3.3%) patients, respectively . In 1 (3.3%) patient of the 2nd group, in addition, clinical signs of vulvovaginal candidiasis were identified.
Normalization of lactobacillary microflora indicators was established in 83.3% of patients in group 1, which significantly exceeded these indicators in patients in group 2 (56.7%). At the same time, the absence of representatives of the Lactobacillus
in laboratory studies, it was observed in 10 (33.3%) patients treated with Trichopolum (metronidazole), and only in 3 (10.0%) patients treated with Tiberal (ornidazole). However, the results obtained may be a consequence of the patient sample and dictate the need for further research to study the effect of drugs on the lactobacillary microflora of the vagina.
Against the background of the therapy, it was possible to achieve a decrease in the intensity of the average severity of pathological discharge (as assessed by patients) by 16.7% in patients of the 1st group, while there was no dynamics in patients of the 2nd group, the unpleasant “fishy” smell of discharge from the genital tract - by 41.2 and 5.9%, respectively, discomfort in the genital tract - by 100% in patients of the 1st group with an increase of 62.5% in patients of the 2nd group, the severity of pain during sexual intercourse - by 100% in patients of the 1st group with an increase in the indicator by 40% in patients of the 2nd group, the severity of pathological discharge according to the doctor’s assessment - by 13.0% in patients of the 1st group with no dynamics in patients of the 2nd group (Fig. 7 and 8).
Rice. 7. Dynamics of the level of severity of symptoms of the disease in patients of group 1 before and after treatment.
Rice. 8. Dynamics of the level of severity of symptoms of the disease in patients of group 2 before and after treatment.
When assessing the overall effectiveness of therapy by the patient, the following results were obtained: “marked improvement” was indicated by 29 (96.7%) patients of the 1st group and 20 (66.7%) patients of the 2nd group, “minor improvement” - 1 (3.3%) and 5 (16.7%) patients, respectively, about “condition without changes” - 3 (10.0%) patients of group 2, about “deterioration” - 2 (6.7%) patients 2nd group.
When assessing the overall effectiveness of therapy by a doctor, clinical recovery was registered in 29 (96.7%) patients of group 1 and 20 (66.7%) patients of group 2, significant improvement - in 2 (6.7%) patients 2 group, improvement - in 3 (10%) patients of group 2, condition unchanged - in 1 (3.3%) patient of group 1 and 2 (6.7%) patients of group 2, deterioration — in 3 (10.0%) patients of group 2.