Home | About us | Delivery | Advertisers | Login | Registration
The pharmacy is closed on Sundays and holidays.
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2021 Pharmacy 84.
Sorbifer Durules No. 50 tab.p.o.
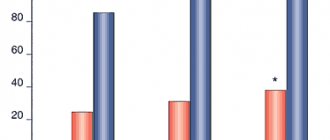
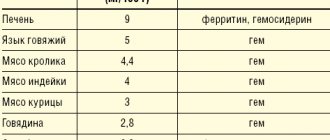
Instructions for medical use of the drug Sorbifer® Durules® Trade name Sorbifer® Durules® International nonproprietary name No Dosage form Film-coated tablets Composition One tablet contains active ingredients: iron (II) sulfate dry 320 mg (equivalent to 100 mg iron (II)) , ascorbic acid 60 mg, excipients: povidone (K-25), polyethene powder, carbomer 934 P, magnesium stearate, shell composition: hypromellose, macrogol 6000, titanium dioxide E 171, iron (III) yellow oxide E 172, solid paraffin . Description Tablets are lenticular-shaped, slightly biconvex, ocher-coated, yellow in color, engraved “Z” on one side, with a characteristic odor. Pharmacotherapeutic group Hematopoiesis stimulants. Iron supplement. Fe++ preparations for oral administration. ATC code B03A A Pharmacological properties Pharmacokinetics "Durules" is a special production technology that ensures the gradual release of the active substance (iron ions), a uniform supply of the drug. Iron is absorbed primarily in the duodenum and proximal jejunum. Taking 100 mg twice a day provides 30% greater absorption of iron from Sorbifer Durules compared to conventional iron supplements. Absorption and bioavailability of iron are high. Bonding with plasma proteins is 90% or more. Deposited in the form of ferritin and hemosiderin in hepatocytes and cells of the phagocytic macrophage system, a small amount is deposited in the form of myoglobin in muscles. The half-life is 6 hours. The presence of ascorbic acid in the preparation creates more favorable conditions for the absorption of iron in the intestines. At the molecular level, ascorbic acid mobilizes iron from the crystalline core of ferritin in vitro, reducing Fe 3+ to Fe 2+. At the intracellular level, ascorbic acid enhances iron-induced ferritin translation by promoting the conversion of iron regulatory protein (IRP) from its RNA-bound form to aconitase. Pharmacodynamics Iron is an essential component of the body, necessary for the formation of hemoglobin and the implementation of oxidative processes in living tissues. The active substance is contained in a biologically indifferent plastic matrix with a spongy structure. When passing through the gastrointestinal tract from the porous matrix of the tablets (within 6 hours), a continuous release of ferrous ions occurs. The film coating of the tablet and the porous matrix provide long-term release of iron ions. The film coating of the tablet, which completely disintegrates under the action of intestinal peristalsis and releases the active ingredient, helps prevent the tablet from dissolving in the stomach. The slow release of iron ions does not lead to the creation of a high local concentration, which avoids irritation of the mucous membrane of the gastrointestinal tract. Ascorbic acid slows down the breakdown of ferritin by blocking autophagy of ferritin by ferritin lysosomes and transformation into hemosiderin. Ascorbic acid accelerates the absorption of iron in the gastrointestinal tract, reducing unbound heme iron (III) to iron (II) in the stomach. Indications for use - iron deficiency anemia - latent iron deficiency in the body (without anemia), associated with excessive iron losses (bleeding, including uterine bleeding, constant donation), malnutrition - conditions accompanied by an increased need for iron in the body (prevention during pregnancy, lactation and in blood donors) Method of administration and dosage The tablet should be taken whole, without chewing, and at least half an hour before meals, with half a glass of water. Adults and adolescents over 12 years of age: Recommended dose: 1 tablet 2 times a day (morning and evening). For patients with grade II-III iron deficiency anemia, if necessary, on the recommendation of a doctor, the dose can be increased to 3-4 tablets 2 times a day (morning and evening) with a duration of use of 3-6 months. During pregnancy: The drug is used in case of established iron deficiency (with iron deficiency anemia and latent iron deficiency in the body) Prophylactic dose: 1 tablet per day. Therapeutic dose: 1 tablet 2 times a day (morning and evening). Treatment should be carried out until hemoglobin levels normalize and continue until the iron depot is completely saturated for another 2 months. Individual long-term therapy (with or without interruptions) is indicated for regularly occurring significant iron loss (for example, with heavy menstruation). Side effects The frequency of side effects from the digestive system increases with increasing doses from 100 to 400 mg per day. Often (>1/100) - nausea, abdominal pain, diarrhea, constipation Rarely (<1/100) - ulcerative lesions of the esophagus, esophageal stenosis - allergic reactions (itching, rash, hyperemia) - hyperthermia Contraindications - hypersensitivity to active or any other inactive component of the drug - esophageal stenosis and/or other obstructive changes in the digestive tract - increased iron content in the body (hemosiderosis, hemochromatosis) - repeated blood transfusions - impaired iron utilization (lead anemia, sideroblastic anemia, hemolytic anemia) - children under 12 years of age (due to lack of clinical data) Drug interactions Concomitant use of Sorbifer Durules with the following drugs is not recommended: - ciprofloxacin: simultaneous use reduces the absorption of ciprofloxacin by approximately 50%, so there is a risk that the content of ciprofloxacin in the blood plasma will be lower than necessary for therapeutic action. - levofloxacin: simultaneous use reduces the absorption of levofloxacin. -moxifloxacin: simultaneous use reduces the bioavailability of moxifloxacin by almost 40%, therefore, if simultaneous use is required, it is necessary to ensure the longest possible period between taking moxifloxacin and Sorbifer Durules. - norfloxacin: simultaneous use reduces the absorption of norfloxacin by approximately 75%. - ofloxacin: simultaneous use reduces the absorption of ofloxacin by approximately 30%. When using Sorbifer Durules concomitantly with the following drugs, dosage adjustment of these drugs may be required. The recommended minimum time interval between taking Sorbifer Durules and these drugs should be at least 2 hours: - calcium or dietary supplements containing magnesium carbonate, as well as aluminum hydroxide or calcium, antacids containing magnesium carbonate, together with iron salts form a complex that reduces absorption each other. - Captopril: Concomitant use reduces the area under the plasma concentration-time curve for captopril by approximately 37%, presumably due to chemical reactions in the gastrointestinal tract. - zinc: simultaneous use reduces the absorption of zinc salts. - clodronate: simultaneous use may reduce the absorption of clodronate. - deferoxamine: with simultaneous use, the absorption of deferoxamine and iron is reduced due to the formation of a compound. - levodopa: when used concomitantly, ferrous sulfate reduces the bioavailability of single doses of levodopa by approximately 50%, and of single doses of carbidopa by 75%, possibly due to the formation of a chelate. - methyldopa: with simultaneous use, the bioavailability of methyldopa is reduced, possibly due to the formation of a chelate. - penicillamine: simultaneous use of penicillamine and iron salts reduces their absorption, possibly due to the formation of a chelate compound. - risedronate: in vitro studies have shown that preparations containing iron form compounds with risedronate. Although in vivo drug interaction studies have not been conducted, concomitant use of these drugs may reduce the absorption of risedronate. - tetracyclines: with simultaneous use, the absorption of tetracyclines and iron is reduced. If simultaneous use is necessary, the recommended minimum time interval between taking Sorbifer Durules and these drugs should be at least 3 hours. When taken orally, iron inhibits the enterohepatic circulation of oxytetracyxine (doxycycline), as well as when administered intravenously. - thyroid hormones: with the simultaneous use of iron and thyroxine preparations, the absorption of thyroxine may be reduced, which reduces the effectiveness of replacement therapy. -cimetidine: with simultaneous use, the decrease in gastric acid production caused by cimetidine reduces the absorption of iron. Therefore, the intervals between taking these drugs should be at least 2 hours. -chloramphenicol: when taken simultaneously, the clinical effect of iron treatment may be delayed. When taking the drug simultaneously with tea, coffee, eggs, dairy products, wheat bread, porridge or foods rich in plant fiber, iron absorption may be reduced. Special instructions Iron supplements can cause poisoning in children. When using the drug, darkening of the stool appears, which has no clinical significance. The drug is used with caution for gastric and duodenal ulcers, inflammatory bowel diseases (enteritis, diverticulitis, ulcerative colitis, Crohn's disease), chronic liver and kidney diseases. During the treatment period, it is recommended to monitor the concentration of hemoglobin and iron in the blood. Comprehensive laboratory and instrumental monitoring of the effectiveness of treatment is recommended to be carried out every 7-14 days, depending on the course of anemia. Pregnancy and lactation The drug can be used during pregnancy and lactation. Features of the effect of the drug on the ability to drive a vehicle or potentially dangerous mechanisms. Does not affect. Overdose Symptoms: abdominal pain, vomiting and diarrhea (sometimes with blood), fatigue, weakness, hyperthermia, paresthesia, pale skin, cold clammy sweat, acidosis, weak pulse, decreased blood pressure, palpitations. Signs of peripheral circulatory collapse, coagulopathy, hyperthermia, hypoglycemia, liver damage, renal failure, muscle cramps and coma may appear after 6-12 hours. Treatment: gastric lavage, milk and raw eggs orally, drugs that provoke vomiting, symptomatic therapy. If necessary, perform a gastric lavage with a solution of deferoxamine at a concentration of 2 g/l, then 5 g of deferoxamine is dissolved in 50-100 ml of water and this solution is left in the stomach. In case of severe intoxication: in a state of shock and/or coma and in case of increased serum iron levels (> 90 mmol/l in children, > 142 mmol/l in adults), intensive care should be started immediately and deferoxamine should be administered (15 mg/l). kg/h intravenously slowly, maximum 80 mg/kg/24 hours). Too high an infusion rate may result in hypotension. In less severe cases of intoxication, deferoxamine can be administered intramuscularly (50 mg/kg, total dose not more than 4 g). It is recommended to monitor serum iron throughout the course of treatment. Release form and packaging 30 and 50 tablets are placed in brown glass bottles, sealed with a polyethylene cap and equipped with an accordion shock absorber for glass bottles. The bottle, together with instructions for medical use in the state and Russian languages, is placed in a lithographed cardboard box. Storage conditions Store at a temperature not exceeding 25 °C. Keep out of the reach of children! Shelf life 3 years Do not use after expiration date. Conditions for dispensing from pharmacies Without a prescription 1106 BUDAPEST, st. Keresturi, 30-38 HUNGARY Tel.: (36-1) 803-5555, Fax: (36-1) 803-5529 Under license from AstraZeneca, Sweden Registration certificate holder JSC "EGIS Pharmaceutical Plant", Hungary Address of the organization receiving the territory of the Republic of Kazakhstan, claims from consumers regarding the quality of products (products) Representative office in the Republic of Kazakhstan CJSC "EGIS Pharmaceutical Plant" 050060, Almaty, st. Zharokova 286 G tel: +, +, fax: + 7 (727) 247 61 41, e-mail
Sorbifer during pregnancy and lactation
Sorbifer during pregnancy is prescribed for anemia .
According to the instructions, in the initial stages of pregnancy (the first six months), it is recommended to take one tablet of the drug once a day. Last trimester and when breastfeeding - 1 tablet twice a day.
Treatment is continued until the level of iron in the blood normalizes. As prescribed by the doctor, the medication can be extended up to 6 months.