Hypertension requires specialist intervention. You cannot let the disease take its course, ignoring constant surges in blood pressure. If blood pressure levels regularly increase, it is necessary to undergo a comprehensive examination, determine the type and degree of hypertension, and obtain from a doctor an individual treatment regimen, which necessarily contains medications that lower blood pressure. Aliskiren is a drug from the group of renin inhibitors. Renin is a hormone that, when in excess, causes an increase in blood pressure. Using tablets you can quickly lower your blood pressure.
Brief information about the medicine
Release form
Aliskiren is produced in the form of white tablets, which are coated with a thin film coating.
The tablets are packaged in individual blister cells of 28 pieces per pack. Aliskiren tablets are available for sale with different amounts of the active substance in the composition - 150 or 300 mg. The kit includes instructions for using Aliskiren.
Compound
The main active ingredient that has a hypotensive effect is aliskiren. One tablet may contain 150 or 300 mg of the substance.
Other components of the drug:
- polyvidone;
- microcrystalline cellulose;
- magnesium stearate;
- iron oxide;
- magnesium silicate;
- hydroxypropyl methylcellulose.
Trade names
In pharmacies, aliskiren can be found under the guise of Rasilez tablets. This is an aliskiren-containing drug that is a complete structural analogue.
Aliskiren is found in pharmacies under the name Rasilez
Manufacturer
The drug is produced in Switzerland.
pharmachologic effect
The selective renin inhibitor Aliskiren has a pronounced hypotensive effect.
Storage conditions and periods
The drug should be stored out of reach of children at temperatures from 0 to 30 degrees Celsius.
The shelf life of the medicine is 2 years from the date of production. After the specified period, the tablets are thrown away.
Conditions for dispensing from a pharmacy
The drug can be purchased at a pharmacy only upon presentation of a prescription from a doctor.
Price
The price of Aliskiren tablets depends on the dosage and varies from 2000 to 2500 rubles.
Pharmacodynamics
A drug from the group of selective renin inhibitors prevents the enzyme angiotensin 1 from being converted into the enzyme angiotensin 2, thereby blocking the production of the enzyme.
When taking aliskiren, the level of renin decreases, which, when excessively produced by the renin-angiotensin-aldosterone system, increases blood pressure. Renin is released by the kidneys when perfusion and circulating blood volume decrease. When entering the circulatory system, renin converts angiotensin into angiotensin 1, which is further converted into angiotensin 2, an enzyme that causes vasoconstriction and the release of catecholamines. Blood pressure is affected by excess angiotensin 2. When renin levels increase, the entire cardiovascular system suffers, which leads to the development of various heart pathologies.
Scheme of action of renin inhibitors
Aliskiren lowers blood pressure, increases renin levels in the blood, and reduces the production of angiotensin enzymes. With prolonged use, the patient's blood pressure stabilizes.
Introduction
The renin-angiotensin-aldosterone system (RAAS) is a key pathophysiological factor in damage to the cardiovascular system in the cardiovascular continuum [1, 2].
Pharmacotherapeutic approaches to the treatment of cardiovascular diseases are based on the use of drugs of different classes that block the activity of the RAAS. These include β-blockers (BABs), angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ATII; ARAs), aldosterone antagonists, and direct renin inhibitors. Currently, two classes of drugs that block the RAAS are the most widely used clinically: ACE inhibitors and ARBs. The purpose of their influence is to eliminate the main effects of ATII - vasoconstrictive and proliferative, leading to unfavorable hemodynamic and structural and functional changes (remodeling) in target organs. However, taking into account the mechanisms of action of ACEIs and ARAs, it is not always possible to block the high activity of the RAAS, which may be the reason for their lack of effectiveness.
It is known that ACE inhibitors and ARBs increase renin activity by a feedback mechanism, which is the reason for the evasion of the effectiveness of RAAS blockers. Renin is the enzyme in the first step in the conversion of angiotensinogen to ATII (Fig. 1), and is also involved in the feedback mechanism. Therefore, inhibition of renin allows for more complete blockade of the RAAS [3]. The search for renin inhibitors has been ongoing since the 1970s; in 2002, the FDA approved the first oral direct renin inhibitor, aliskiren.
Aliskiren has a selective tropism for plasma renin: it binds the catalytic center of renin activity, does not reduce its concentration in plasma, but reduces its activity. As a result, the formation of ATII is reduced by 80%, and the level of other forms of AT, which also have a certain biological activity, decreases (Table 1) [4, 5].
Thus, in recent years, much attention has been paid to studying the role of these substances in the regulation of vascular and proliferative effects associated with the RAAS. Alternative non-renin pathways for the formation of ATI and ATII were discovered through the tissue form of angiotensin 1–12 and an alternative ACE2 (AT1-7) MAS system, which acts as a counterbalance to the level of ATII (Fig. 2) [5]. ACE2 performs a function opposite to that of ACE: it participates in the alternative pathway of ATII degradation in AT1-7 and, to a lesser extent, ATI in AT1-9. It has been shown that ACE2 plays a dominant role in the regulation of ATII levels in tissues (heart, kidneys, lungs, gastrointestinal tract); it is not affected by ACE inhibitors. The activities of ACE and ACE2 determine the balance between the pressor-proliferative and depressor-antiproliferative components of the system, which controls cardiovascular homeostasis. AT1-7 interacts with its own receptors – AT3 and MAS, as well as with AT2 receptors, mediating vasodilation and antiproliferation. It is emphasized that the AT1-7/ATII balance is important for maintaining cardiovascular homeostasis [6]. Aliskiren, which blocks renin activity, reduces the levels of ATI and ATII in plasma, but does not affect the alternative (non-renin) pathway of peptide formation in tissues; as a result, the AT1-7/ATII balance may be disrupted by reducing the level of AT1-7 [7].
In addition, renin can interact with previously discovered prorenin receptors and have an independent effect on the down-regulation of key peptides of the RAAS system. Aliskiren affects the expression of the prorenin receptor gene in the kidneys (but not in the myocardium and vascular wall), which explains an additional independent mechanism for the development of the beneficial effects of the drug in kidney diseases [5, 7].
Antihypertensive effectiveness
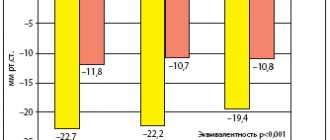
According to a pooled analysis of 7 multicenter placebo-controlled clinical trials (CTs), in 7045 patients with stage 1-2 arterial hypertension (AH), aliskiren at a dose of 150 mg/day led to a decrease in systolic blood pressure (SBP) by 8.7 –13.0 mmHg and diastolic blood pressure (DBP) by 7.8–10.3 mm Hg, and at a dose of 300 mg/day – by 14.1–15.8 and 10.3–12.3 mm Hg. respectively [8].
In recent years, a large number of comparative CTs have been conducted to study the antihypertensive effectiveness of aliskiren, the results of which are presented in a number of systematic reviews and meta-analyses. First of all, the effects of aliskiren and ARAs were compared both in monotherapy and in combinations. In a meta-analysis by Z. Zheng et al. 7 CTs (n=5488) lasting 4–8 weeks were included, in which aliskiren was prescribed in doses of 150–300 mg/day, ARA drugs (losartan, valsartan, irbesartan) – in half and maximum doses [9]. We assessed the degree of reduction in SBP and DBP, the frequency of achieving target BP, and the tolerability of therapy based on the incidence of side effects (Table 2). There were no significant differences in the severity of the antihypertensive effect between aliskiren and ARA drugs; There were no differences in the level of blood pressure control in patients with hypertension. At the same time, the combination of aliskiren with ARAs caused a significantly more pronounced decrease in blood pressure and increased the frequency of achieving the target blood pressure level by almost one and a half times. Tolerability studies did not show significant differences between the drugs in all cases of side effects, individual reactions, or in the frequency of withdrawal due to side effects. However, there was a statistically significant increase in the incidence of weakness and hyperkalemia with the combination of aliskiren and ARA.
The results of a meta-analysis by D. Gao et al. were published, combining 10 comparative CTs (n=3732) of aliskiren with ARB drugs (losartan, valsartan, irbesartan) lasting 4–12 weeks; the drugs were used in equivalent therapeutic doses as monotherapy [10]. There were no significant differences in the degree of blood pressure reduction between aliskiren and ARA drugs, either during office blood pressure measurement (Table 3) or during ambulatory blood pressure monitoring. In monotherapy with aliskiren and ARAs, the rates of achieving the target blood pressure level were also comparable, and no advantages of aliskirin were found over any drug of the ARA class (RR about 1.0). Tolerability of the drugs also did not differ.
The effectiveness of aliskiren was also compared with other antihypertensive drugs. Thus, in a meta-analysis by Y. Chen et al. (14 CTs, n=6741) aliskiren was compared with ARBs (losartan, valsartan, irbesartan), ACE inhibitors (ramipril, lisinopril), calcium antagonist (amlodipine), beta blocker (atenolol) and diuretic (hydrochlorothiazide), as well as with placebo [11] . In terms of antihypertensive efficacy, aliskiren turned out to be comparable to ARA, as in previous meta-analyses, and hydrochlorothiazide, superior to ACE inhibitors and beta blockers, but inferior to the calcium antagonist amlodipine (Table 4).
The problem of comparing the antihypertensive effectiveness of aliskiren with other drugs arose due to reports of a paradoxical increase in blood pressure during long-term therapy (escape of the effect), which may be associated with a reactive increase in plasma renin concentrations [12]. To study this issue, AV Stanton et al. conducted a special meta-analysis of 8 placebo-controlled CTs (n=4877), which used aliskiren, ARB drugs (losartan, valsartan, irbesartan), ACE inhibitor (ramipril) or hydrochlorothiazide in therapeutically equivalent doses and assessed the incidence of blood pressure increases (according to SBP criteria >10 and DBP>5 mmHg) against the background of monotherapy after 4–12 weeks [13]. The incidence of paradoxical increases in SBP and DBP during the use of aliskiren was not statistically higher than that when using other classes of antihypertensive drugs that can activate plasma renin with long-term use, and was significantly lower compared to the placebo group (Table 5). Moreover, in none of the 536 patients receiving aliskiren at a dose of 300 mg/day, an increase in blood pressure was associated with an increase in plasma renin activity by 0.1 ng/mLh.
Thus, aliskiren demonstrates high clinical efficacy in the treatment of patients with stage 1–2 hypertension, having good tolerability and a similar frequency of side effects with other antihypertensive drugs.
Organoprotective and long-term clinical effects
The study of the broader possibilities of using aliskiren in the treatment of cardiovascular diseases is planned in the large-scale research program ASPIRE HIGHER, consisting of 14 CTs involving 35 thousand patients (Table 6) [14].
A number of clinical trials have shown organoprotective effects of the drug: regression of LVH in patients with hypertension (ALLAY) [15], antiproteinuric effect in patients with hypertension and diabetic nephropathy (AVOID) [16].
Thus, in the ALLAY study in patients with hypertension and LVH (thickness of the posterior wall of the left ventricle more than 1.3 cm according to ECHO-CG), the use of aliskiren was associated with a similar degree of regression of the LVMM index compared with losartan and the combination of aliskiren with losartan: by 5.7 ±10.6, 5.4±10.8 and 7.9±9.6 g/m2, respectively [15]. In some patients (n = 136), the dynamics of RAAS neurohormones was studied, and a significant and significant decrease in the level of aldosterone and plasma renin activity was revealed during the use of aliskiren or a combination of aliskiren with losartan, while during the use of monotherapy with losartan there was no effect on aldosterone, and on renin activity was the opposite.
In the AVOID study, patients with hypertension and diabetes with proteinuria after 3 months of treatment with losartan at a dose of 100 mg/day and achieving the target blood pressure level (<130/80 mm Hg) with a compensated level of glycemia (glycated hemoglobin - 8%) were randomized to taking aliskiren in doses of 150–300 mg/day or placebo. There was a significant decrease in the urine albumin/creatinine index (primary endpoint) by 11% after 3 months and by 20% after 6 months compared with the placebo group (p<0.001) against the background of a comparable decrease in blood pressure; the proportion of patients with a decrease in urinary albumin/creatinine index of 50% or more was twice as large compared with placebo (24.7 vs. 12.5%; p < 0.0005) [16]. Moreover, the nephroprotective effect of aliskiren was not associated with a decrease in blood pressure. One of the explanations for the nephroprotective effect of aliskiren is the experimental data previously obtained on DM models about the ability of the drug to reduce the number of renin and prorenin receptors in the kidneys, as well as weaken profibrotic processes and podocyte apoptosis, which provides a more pronounced effect compared to ACE inhibitors [17].
In the ALOFT study in elderly patients with CHF and hypertension, aliskiren at a dose of 150 mg was added to standard therapy (ACE inhibitors or ARBs, beta blockers) [19]. The primary endpoint assessed the tolerability and safety of aliskiren in CHF: there were no differences in the incidence of deterioration in renal function, hyperkalemia and hypotension in the alskiren group compared with standard therapy. The addition of aliskiren to standard therapy was accompanied by a significant decrease in the levels of BNP, NT-proBNP and aldosterone in the urine. It is precisely this dynamics of BNP and NT-proBNP that is associated with an improvement in long-term prognosis in patients with CHF.
The AVANT-GARDE study examined the dynamics of NT-proBNP after 8 weeks (primary endpoint) in patients with ACS and preserved EF while adding aliskiren to standard therapy [20]. The decrease in NT-proBNP levels was 42, 44, 39 and 36% in the placebo, aliskiren, valsartan and aliskiren + valsartan combination groups, respectively. When studying the dynamics of aldosterone levels, its increase in these groups was noted by 19.7, 10%, 9.9 and 9.3%, respectively; the degree of increase in aldosterone levels was significantly less during the use of RAAS blockers. There were no differences in the incidence of clinical outcomes between groups. However, a higher incidence of serious side effects (hypotension, hyperkalemia) was detected in all groups compared to placebo.
The ASPIRE study on 820 patients with AMI examined the effectiveness of aliskiren in preventing post-infarction remodeling [21]. There was no additional effect in reducing LV EDV (primary endpoint) after adding aliskiren to standard therapy (-4.4 vs. -3.5 ml in the placebo group), as well as in relation to EF dynamics and clinical outcomes. However, with the use of aliskiren, a significantly higher incidence of side effects was noted, especially cases of hypotension, renal dysfunction and hyperkalemia.
In 4 specially planned large clinical trials, the long-term clinical effects of aliskiren were studied: in patients with hypertension and a high risk of cardiovascular complications (APOLLO, ALTITUDE) and patients with CHF (ASTRONAUT, ATMOSPHERE). The results of three completed CTs did not reveal any advantages of the drug in combination with standard therapy (including combination with ACE inhibitors/ARBs) in the prevention of CV events, AMI, stroke, and progression of CHF, but demonstrated worse safety data [14]. The results of the latest ATMOSPHERE trial have not yet been published. The results obtained can be explained by the peculiarities of the proximal blockade of the RAAS, as a result of which there is a decrease in the formation of not only ATII and ATIII, which have an adverse effect on cardiovascular homeostasis, but also AT1-7, which helps maintain balance in it.
The AQUARIUS study examined the possibility of the effect of aliskiren on the progression of coronary atherosclerosis in 592 patients with coronary artery disease [22]. The dynamics of changes in atheroma volume (primary endpoint), both in percentage terms and in absolute value, were not statistically different between the aliskiren and placebo groups (-0.33 versus 0.11% and -4.1 versus -2.1 mm3, respectively) ; There were no significant differences in the proportion of patients with regression of atheroma (64.4 versus 57.5%, respectively). At the same time, in the aliskiren group there was a lower frequency of main adverse clinical outcomes: the frequency of the first cardiovascular outcome was observed 2 times less often than in the control group (OR = 0.50; p = 0.004); cases of AMI were also observed statistically less frequently (OR=0.13; p=0.02). Therefore, the possibility of a protective effect of aliskiren on the atherosclerotic process requires further study.
Safety and side effects
According to the results of meta-analyses of clinical trials, the use of aliskiren as monotherapy in patients with hypertension is well tolerated, and its safety is comparable to placebo [9–11]. These data were confirmed in a specially performed meta-analysis by WB White et al. (12 CTs, n=12,188), which compared the incidence of side effects when using aliskiren and antihypertensive drugs of other groups in patients with hypertension [23]. The overall frequency of side effects and the frequency of development and serious side effects of aliskiren in long-term clinical trials did not differ significantly from those of drugs in other groups (Table 7).
However, the combination of aliskiren with other RAAS blockers, as well as the combination of two RAAS blockers (ACEI and ARB), especially for patients with concomitant renal impairment, may be accompanied by an increased risk of serious side effects: renal dysfunction, hyperkalemia, hypotension.
In a meta-analysis by Z. Harel et al. (10 trials, n=4814) obtained generalized data on the frequency of side effects of combination therapy with aliskiren and ACEI/ARB compared with monotherapy with aliskiren or ACEI/ARB [24]. The risk of developing renal dysfunction (serum creatinine >176.8 mmol/l) when using a combination of aliskiren with ACEI/ARB compared with aliskiren monotherapy did not increase, but compared with ACEI/ARB monotherapy it increased by 17%, although statistical significance of the differences was not achieved. was (Table 8). The risk of hyperkalemia (>5.5 mmol/l) was significantly higher when using a combination of aliskiren with ACEI/ARB compared to monotherapy with any RAAS blocker, mainly due to cases of moderate hyperkalemia (5.55.9 mmol/l).
Another meta-analysis assessed the overall safety and risk of side effects when using a combination of aliskiren with an ACEI/ARB or a combination of an ACEI with an ARB compared with RAAS blocker monotherapy based on the results of 50 clinical trials [25]. Thus, the risk of overall mortality and cardiovascular events when using a combination of drugs that block the RAAS did not increase; at the same time, the risk of developing renal dysfunction, hyperkalemia and hypotension significantly increased by more than 50%, regardless of the inclusion of aliskiren in the combination (Table 9).
Thus, the use of a combination of drugs that block the RAAS is accompanied by serious undesirable effects on renal function and a sharp increase in the risk of hypotension, which requires absolute caution to ensure safety.
Conclusion
Alisicren is another drug that blocks the RAAS and is an effective antihypertensive agent comparable in effectiveness and safety to drugs of other classes. In recent years, the evidence base regarding the effect of aliskiren has been significantly supplemented by the results of a large number of large clinical trials. They show that when used as monotherapy, alisicrene provides a pronounced antihypertensive effect and is well tolerated by patients with hypertension of different age groups; can be combined with other antihypertensive drugs (diuretics, calcium antagonists), except ACE inhibitors/ARBs. Large studies have shown the organoprotective effects of aliskiren - regression of LVH in patients with hypertension and proteinuria in patients with hypertension and diabetic nephropathy. A study of the long-term effectiveness of aliskiren for patients with hypertension did not reveal a worsening prognosis (cardiovascular and overall mortality). However, a study of the possibilities of using aliskiren in other cardiovascular diseases (CHF, ACS, AMI, IHD) did not reveal any particular clinical advantages of the drug.
When use is contraindicated
Absolute contraindications
Contraindications to the use of Aliskiren for high blood pressure:
- nephrotic syndrome;
- hypertension caused by renal artery occlusion;
- severe renal dysfunction;
- severe liver dysfunction;
- individual intolerance to components;
- hemodialysis;
- children under 18 years of age;
- pregnancy;
- lactation.
For patients requiring hemodialysis, the drug is prohibited
Use with caution
Aliskiren should be taken with caution if you have high blood pressure in the following cases:
- diabetes;
- stenosis or occlusion of the renal arteries;
- lack of sodium;
- decreased circulating blood volume;
- excess potassium.
Main contraindications for taking Aliskiren
The drug has an intense effect on human organs and can affect the course of certain pathologies. In this regard, the official instructions for the drug highlight conditions that prohibit the use of Aliskiren. A number of contraindications:
- minor age group;
- lactation and pregnancy;
- allergic reactions and hypersensitivity to the substances included in the composition;
- severe pathological processes in the genitourinary system;
- nephrotic syndrome;
- systematically undergoing hemodialysis;
- dangerous, severe disruptions in liver function;
- arterial hypertension of the kidneys.
Aliskiren is taken only under medical supervision and with constant monitoring of vital signs by patients with a medical history of the following pathologies:
- chronic endocrine disorders;
- diabetes;
- reduction of blood volume in the circulating bloodstream;
- severe hyperkalemia;
- disturbances in the blood supply to the kidneys.
Contraindications
It should be noted that Aliskiren has a number of contraindications, so it is prescribed by a doctor.
Use during pregnancy and breastfeeding
The drug penetrates the placental barrier and can cause significant harm to the fetus developing in the womb. Aliskiren has a negative effect on the renin-angiotensin system, which can lead to fetal death before or immediately after birth. The use of tablets increases the risk of developing congenital pathologies.
When planning a pregnancy, you should stop taking Aliskiren and work with your doctor to find a safer method of treating hypertension.
When taking a course of the drug, it is necessary to take care of reliable methods of contraception to prevent the occurrence of accidental pregnancy.
If a woman becomes pregnant during a course of treatment, therapy should be interrupted as quickly as possible to reduce the risk of negative effects on the fetus.
There is insufficient data on the extent of penetration of aliskiren into breast milk. Doctors recommend stopping breastfeeding for the entire period of taking the pills.
Aliskiren is strictly contraindicated for pregnant women and nursing mothers
Instructions for use and dosage
The drug is used orally, regardless of food intake. The tablets are drunk whole with water. The medicine can be prescribed as a monotherapy for the treatment of hypertension or as part of complex therapy with other antihypertensive drugs.
A drug with a cumulative effect requires a course of use. A single use of the drug will not have the required antihypertensive effect. A visible hypotensive effect is observed two weeks after the start of therapy.
If you have high blood pressure, you should take Aliskiren 150 mg once a day. If after two weeks of taking the indicated dosage there is no therapeutic effect, the dose is increased to 300 mg per day. The maximum daily dosage is 300 mg.
In case of kidney transplantation and narrowing of the lumen of the renal arteries, the drug is prescribed in a minimal dose. It is impossible to increase the dosage in this condition.
If the patient has mild renal or liver dysfunction, no dose adjustment is required.
Elderly patients do not need to change the daily dosage.
Special rules for using the drug
The guidelines for the use of Aliskiren require compliance with specific administration recommendations. These include:
- Initial use in a hospital setting if the patient has the following diagnoses: diabetes mellitus, renal artery stenosis, organ transplantation, hyperkalemia, hyponatremia.
- In case of disorders in the endocrine system or diabetes mellitus, it is important to monitor the levels of electrolytes in the blood and control the performance of the genitourinary system.
- The occurrence of acute allergic reactions is a reason to stop taking Aliskiren.
- In case of hyperkalemia, hyponatremia, before starting therapeutic action, it is important and necessary to normalize the water-salt balance.
Please note that the drug has a strong effect, and compliance with the above recommendations is mandatory.
Side effects
Side effects of the drug are caused by the body's reaction to the components. They manifest themselves differently in all patients:
- loose stools;
- rash;
- signs of allergy in case of intolerance to components.
In isolated cases, a decrease in hematocrit value and hemoglobin level was observed.
Drug intolerance may result in a skin rash
Drug interactions
Aliskiren does not have a strong effect on other medications, with the exception of Cyclosporine and Furosemide. When taking Cyclosporine simultaneously with Aliskiren, a fivefold increase in the concentration of the active components of Cyclosporine is possible. Complex therapy is excluded.
The simultaneous use of Furosemide with Aliskiren leads to a decrease in the content of the active substances of Furosemide several times, so complex therapy requires adjustment of the daily dose.
Caution must be exercised when prescribing Aliskiren for high blood pressure with the following medications:
- potassium-sparing diuretics;
- potassium salts;
- salt substitutes;
- drugs that affect the concentration of potassium in the blood.
When taking these drugs with Aliskiren, hyperkalemia may develop.
Aliskiren may be incompatible with some drugs
Indications for use
The drug has a narrow therapeutic effect and is used to treat hypertension when blood pressure systematically exceeds normal criteria. The upper threshold of the acceptable value is 140 to 90.
In rare cases, Aliskiren is used to prevent hypertension among patients with a genetic predisposition to myocardial infarction.
Please note that in the absence or improper treatment of hypertension, the risk of heart attack, heart failure, and death increases several times.
What to replace with arterial hypertension
Medicines containing aliskiren
Aliskiren is part of the antihypertensive drug Rasilez.
Analogues in action
Aliskiren's analogues:
- Valsartan;
- Verapamil;
- Guanfacine;
- Dipivefrin;
- Indapamide;
- Isradipin;
- Clonidine;
- Co-Rasilez;
- Lacidipine;
- Levamlodipine;
- Lercanidipine;
- Moxonidine;
- Moexipril;
- Olmesartan;
- Perindopril;
- Rasilez Duo;
- Reserpine;
- Rilmenidine;
- Telmisartan;
- Trandolapril;
- Triamterene;
- Fimasartan;
- Enalapril;
- Eprosartan.
You cannot independently adjust the treatment regimen by replacing Aliskiren with analogues. The decision to prescribe an analogue is made by the attending physician.