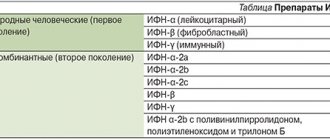
Pharmacodynamics and pharmacokinetics
The substance has the ability to inhibit the process of phosphokinase , stopping the processes of phosphorylation in virus-infected cells. The product completely and irreversibly inactivates the shingles and herpes when they are outside the cells. The penetration of active virus particles into healthy cells is blocked, and the harmful agent loses the ability to synthesize new structural components.
When used topically, the compound has the ability to accumulate in affected cells, while practically not undergoing systemic absorption.
Effective use of glycyrrhizic acid for viral infections of the genital organs
Viral infections of the genital organs continue to attract close attention from researchers and clinicians due to increasing incidence, significant contagiousness and potential oncogenicity.
Figure 1. Dynamics of cytokine levels in the cervical canal after the use of glycyrrhizic acid/placebo for genital herpes
Figure 2. Dynamics of MMP-1 and TIMP-1 levels in the cervical canal after the use of glycyrrhizic acid/placebo for genital herpes
Figure 3. Dynamics of cytokine levels in the cervical canal after the use of glycyrrhizic acid/placebo for papillomavirus infection
Figure 4. Dynamics of the level of MMP-1 and TIMP-1 in the cervical canal after the use of glycyrrhizic acid/placebo for papillomavirus infection
Figure 5. Dynamics of cytokine levels in the cervical canal after the use of glycyrrhizic acid/placebo for cytomegalovirus infection
Figure 6. Dynamics of the level of MMP-1 and TIMP-1 in the cervical canal after the use of glycyrrhizic acid/placebo for cytomegalovirus infection
Research in recent years has shown that viruses are capable of supporting the inflammatory process in the affected area with changes in the structural and functional characteristics of tissue, causing changes in the antigenic structure of infected cells, inducing the appearance of autoantibodies, and can be inducers of neoplastic transformations. Local changes in the mucous membranes and skin during viral invasion are manifested by the appearance of inflammatory infiltrates in the tissue, activation of immune system cells, changes in the level of cytokines, growth factors and proteolytic enzymes, activation of angiogenesis, and changes in gene expression.
Licorice root Glycyrrhiza glabra –
one of the most famous medicines in the world, which was known to the Sumerians and is a classic remedy in Chinese and Tibetan medicine. Hippocrates mentioned it in his works, Theophrastus described it as “a Scythian root from the Sea of Azov.” Overall, licorice has been used as a medicinal remedy for over 5,000 years. In the late 70s of the last century, it was first shown that a substance isolated from licorice root suppresses the activity of viruses. The most active substance of licorice root can be called glycyrrhizic acid, which is similar in structure to steroid hormones, in particular to the phenanthrene part of steroids.
Licorice preparations have multifaceted biological activity and have anti-inflammatory, antiviral and immunomodulatory effects. The anti-inflammatory effect consists of stopping inflammatory reactions caused by histamine, serotonin and bradykinin. The acid inhibits the activity of phospholipase A and the formation of prostaglandin E2 in activated macrophages, accelerates the migration of leukocytes to the inflammatory zone. In this case, both the exudative and proliferative phases of the inflammatory process are inhibited. Studies have shown that glycyrrhizic acid also has a pronounced antiviral effect. The first study of the antiviral properties of glycyrrhizic acid was carried out in 1979, and in the 90s it was found that it interrupts viral replication in the early stages by inhibiting P kinase.
A number of studies have shown that mutant strains of viruses resistant to acyclovir retain sensitivity to glycyrrhizic acid. When acid interacts with virions, various phases of the viral cycle change, which is accompanied by irreversible inactivation of viral particles that are in a free state outside the cells; the introduction of active viral particles into the cell is blocked, the ability of the virus to induce the synthesis of new viral particles is disrupted.
Glycyrrhizic acid also has a pronounced immunomodulatory effect. The ability of glycyrrhizic acid to induce the formation of IFN and activate nonspecific factors of cellular immunity has been demonstrated.
In order to study the local effect of glycyrrhizic acid ( Glicyrriza
glabra ,
Epigen-intim drug, Cheminova) in viral infections of the genital organs, the study included 100 women aged 17 to 40 years, the presence of infection in whom was proven by detection in the genital tract viral DNA by polymerase chain reaction.
All patients were divided into groups:
- Group I – 30 women with human papillomavirus infection of the genital organs;
- Group II – 30 women with a typical form of genital herpes (mild and moderate severity);
- Group III – 30 women with cytomegalovirus infection of the genital organs;
- control group – 10 healthy women of reproductive age.
The study used a monoinfection and monotherapy model to evaluate the mechanism of action of the study drug. In each group, patients received local therapy with glycyrrhizic acid (n = 20) or a placebo drug (n = 10) for 1 month, the drug was applied daily 2-3 times to the mucous membranes of the genital organs by spraying, when lesions were localized on the cervix the drug was applied intravaginally using a special nozzle. Assignment of the drug or placebo in the groups was carried out in a blind manner. No other antiviral drugs were administered during the study. The clinical effectiveness of therapy was assessed during treatment; the follow-up period was 6 months.
In order to assess the local effect of glycyrrhizic acid (before treatment and immediately after its completion), the cervical mucus was examined for the content of pro-inflammatory Th1 cytokines –
IL-1b, IL-6, TNF-α and IFN-γ, anti-inflammatory Th2 cytokines – IL-4, IL-10, as well as matrix metalloproteinases MMP-1 and tissue inhibitors of matrix metalloproteinases TIMP-1.
Mucus from the cervical canal was taken with a universal probe, shaken on a vortex for 1 minute, and washed off in 1 ml of phosphate-saline solution. Then it was centrifuged for 3 minutes. at 3000 rpm. The supernatant was immediately frozen and stored at 80ºC until reactions were performed. In samples of mucus from the cervical canal, the content of IL-1b, IL-6, IL-10, IFN-g, TNF-a, IL-4 (Cytokine, Russia), as well as tissue MMP-1 and TIMP-1 (Chemicon, USA). The levels of factors in cervical mucus were normalized by the protein content in the samples, which was determined by the colorimetric method using bicinchoninic acid (Sigma, USA).
Analysis of the dynamics of clinical symptoms of infection after the use of glycyrrhizic acid indicates the high clinical effectiveness of the drug. In the typical form of genital herpes, during treatment, the duration and clinical severity of relapses of herpes, according to the subjective assessment of patients, decreased significantly, which had a positive effect on the quality of life of patients. Compared with placebo, the use of glycyrrhizic acid resulted in rapid relief of symptoms of vulvodynia (itching, burning, discomfort in the area of herpetic eruptions of the vulva and vagina); faster healing of lesions (on average 2 ± 0.78 days compared to placebo); acceleration of epithelization processes of affected areas; significant reduction in the zone of hyperemia around herpetic elements and the reaction of regional lymph nodes. During treatment, a significant reduction in the local inflammatory reaction surrounding herpetic elements and acceleration of tissue repair processes helped reduce the risk of secondary bacterial infection and the development of complications.
During therapy with glycyrrhizic acid, patients with exophytic forms of papillomavirus infection of the genitals experienced relief of symptoms of persistent discomfort, itching and burning in the external genital area 4-6 days from the start of drug use. In the presence of small and multiple condylomas of the vulva and vagina, a significant decrease in exophytic formations in the size and total area of the lesion was noted. With cytomegalovirus infection, initially only 6 patients had symptoms of vulvodynia, which were quickly relieved by therapy with glycyrrhizic acid.
The main objective of the study was to assess the effect of glycyrrhizic acid on local molecular biological processes in the reproductive tract during viral infections. When analyzing the content of pro-inflammatory cytokines IL-1b, IL-6, TNF-α and IFN-γ in the cervical canal before treatment in all groups, it was revealed that the persistence of the viral infection was accompanied by a significant increase in the level of Th1 cytokines, which reflected the intensity of inflammatory reactions in the tissue and was an important marker of the activation phase of the viral infection. In all types of viral infection, local levels of Th1 cytokines significantly exceeded control values:
- with genital herpes in group I, the level of IL-1b was increased by 33.2 times, IL-6 by 3.5 times, TNF-α by 4.6 times and IFN-γ by 3.6 times;
- with human papillomavirus infection in group II, the level of IL-1b was increased by 62.4 times, IL-6 by 2.4 times, TNF-α by 13.6 times and IFN-γ by 3 times;
- with cytomegalovirus infection in group III, the level of IL-1b was increased by 18.6 times, IL-6 by 1.3 times, TNF-α by 3.7 times and IFN-γ by 2.8 times (in all cases p
The levels of regulatory anti-inflammatory Th2 cytokines IL-4 and IL-10 in all groups before treatment were lower than control values, which indicates virus-induced inhibition of the anti-inflammatory tissue defense system, however, these changes were not significantly significant - p > 0.05 (Figures: 1, 3, 5).
When analyzing the system of tissue proteolytic enzymes, matrix metalloproteinases and their inhibitors as key effectors of tissue remodeling and important markers of the inflammatory process, an increase in the local activity of MMP-1 was revealed in all groups (Figures: 2, 4, 6).
After a course of therapy with glycyrrhizic acid, local levels of Th1 cytokines IL-1b, IL-6, TNF-α and IFN-γ in the cervical canal significantly decreased to control values, while the dynamics of changes were especially indicative in group I of patients with genital herpes, where the initial intensity of inflammatory reactions was maximum (Figures 1, 3 and 5).
Therapy with glycyrrhizic acid led to an increase in the protective potential of the mucous membrane - to the activation of anti-inflammatory Th2 cytokines, while the levels of IL-4 and IL-10 increased, in some cases exceeding control values. When using placebo, the levels of pro-inflammatory Th1 cytokines and regulatory Th2 cytokines fluctuated slightly due to the undulation of the viral infectious process itself, but did not change significantly (p > 0.05 in all groups compared with baseline values and control).
When analyzing the effect of glycyrrhizic acid on the level of tissue matrix metalloproteinases, a significant decrease after treatment in the activity of proteolytic enzymes to control values was revealed:
- in group I, the level of MMP-1 decreased by 2.25 times (p
- in group II – 1.9 times (p
- in group III, the level of MMP-1 decreased slightly (p > 0.05).
Therapy with the drug led to an increase in the protective properties of the mucous membrane - an increase in the level of tissue inhibitors of metalloproteinases to control values was detected: the level of TIMP-1 significantly increased in groups I and II (p
During the use of placebo in all groups, further progression of disorders in the MMP-1/TIMP-1 system was noted with activation of matrix metalloproteinases and a decrease in the content of tissue inhibitors of matrix metalloproteinases.
Discussion
Chronic persistence of viral agents in the mucous membranes leads to disruption of mediator interactions, changes in tissue angioarchitecture, activation of growth factors, which, in turn, activate the processes of cell proliferation and angiogenesis. When mediator interactions and the cellular ensemble are disrupted, the immune barrier of the mucous membranes and skin is incomplete, which leads to the activation of opportunistic flora, the addition of a secondary infection and, ultimately, to an increase in the inflammatory process.
The use of glycyrrhizic acid during viral invasion led to normalization of the local ratio of Th1 and Th2 type cytokines: the levels of key pro-inflammatory cytokines IL-1, IL-6, TNF-α and IFN-γ decreased to control values, the levels of anti-inflammatory cytokines IL-4, IL- 10 increased, which proves the pronounced anti-inflammatory and immunomodulatory effect of the drug. At the same time, the normalizing effect of glycyrrhizic acid on the system of matrix metalloproteinases and their inhibitors was revealed, with a decrease in the pathological lytic potential of infectious agents. Thus, the anti-destructive and protective effects of the drug are shown.
In general, glycyrrhizic acid can be successfully used in the complex treatment of viral infections of the genitals. The use of the drug is permitted during pregnancy and lactation.
Glycyrrhizic acid, instructions for use (Method and dosage)
The dosage regimen is determined individually.
The spray is used for external use. The drug is sprayed for some time (several seconds) on the affected surface at a distance of 5 cm. Frequency of application - up to 6 times a day.
The duration of treatment is from 5 to 10 days.
Vaginally, the drug is prescribed 3-4 times a day, for one week - 10 days. After 10 days, it is recommended to repeat the course again.
The cream is applied to the area affected by the virus 3-5 times a day for a week or 10 days.
Clinical studies of glycyrrhizic acid
Glycyrrhizic acid (GA) is the main active component of licorice root, which has been successfully used in medicine for more than 3,000 years.
To date, the effectiveness and safety of GCs in liver diseases have been well studied.
Nevertheless, scientific interest in this substance remains and the search for new promising areas of its application in medicine continues. Thus, the largest medical information database, PubMed, contains more than 1,400 publications on GC. Among them are the results of 54 clinical studies, 31 of which are randomized, that is, they represent the “gold standard” of drug research. Clinical trial results
A randomized clinical trial of GC in non-alcoholic fatty liver disease (NAFLD), which included 66 patients, demonstrated that it significantly reduced liver inflammation by reducing alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
Similar results were obtained in 12 randomized clinical trials of GC for alcoholic liver disease with a total of 838 participants.
In 2 randomized clinical trials in 436 patients with CHC after ineffective antiviral therapy (AVT), a positive effect of GC on inflammation was shown (a significant decrease in ALT levels). And one of them also showed a decrease in the degree of fibrosis.
In addition, in a retrospective study of 1249 patients, it was found that long-term administration of GC (over several years) for CHC, in case of impossibility of cure with antiviral drugs, significantly reduces the incidence of the most serious complication of this disease - primary liver cancer.
In all of these studies, the safety profile of GCs was assessed as favorable.
The available evidence base has led to the inclusion of GC in the recommendations of the Asia-Pacific Association for the Study of the Liver (APASL) and the approval of GC for medical use in humans by the European Medicines Agency (EMA).
Phosphogliv* is a drug based on glycyrrhizic acid and essential phospholipids
Phosphogliv* is a means of pathogenetic therapy. Its action is aimed at correcting liver dysfunction. The drug contains essential phospholipids and the glycyrrhizic acid described above.
Essential phospholipids have a membrane-stabilizing effect, promoting the restoration of damaged liver cells.
Glycyrrhizic acid in the drug has anti-inflammatory, antioxidant and antifibrotic effects. Thanks to it, the product shows a high degree of effectiveness not only in the treatment of inflammation, but also for prevention purposes.
Ipatova O.M. Phosphogliv: mechanism of action and clinical use. Ed. Academician of the Russian Academy of Medical Sciences Archakov A.I. M.: Publishing house. State Research Institute of Biomedical Chemistry of the Russian Academy of Medical Sciences, 2005. p. 318.1
Ali Akbar Hajiaghamohammadi, Amir Ziaee, Rasoul Samimi. The Efficacy of Licorice Root Extract in Decreasing Transaminase Activities in Non-alcoholic Fatty Liver Disease: A Randomized Controlled Clinical Trial // Phytotherapy Research. 2012; 26(9):1381–1384.2
Community herbal monograph on Glycyrrhiza glabra L. and/or Glycyrrhiza inflata Bat. and/or Glycyrrhiza uralensis Fisch., radix. Committee on Herbal Medicinal Products (HMPC). 22 May 2012. EMA/HMPC/571119/2010.3
Kenji Ikeda, Yasuji Arase, Masahiro Kobayashi et al. A Long-Term Glycyrrhizin Injection Therapy Reduces Hepatocellular Carcinogenesis Rate in Patients with Interferon-Resistant Active Chronic Hepatitis C: A Cohort Study of 1249 Patients // Digestive Diseases and Sciences. 2006; 51(3):603–609.4
M. P. Manns, H. Wedemeyer, A. Singer et al. Glycyrrhizin in patients who failed previous interferon alpha-based therapies: biochemical and histological effects after 52 weeks // Journal of Viral Hepatitis. 2012; 19: 537–546.5
Masao Omata, Tatsuo Kanda, Ming-Lung Yu et al. APASL consensus statements and management algorithms for hepatitis C virus infection // Hepatol Int. DOI 10.1007/s12072-012-9342-y.6
Meng Wei,Yu Liang-zhu,Wang Li. Effects of Compound Glycyrrhizin on Liver Function in Patients with Alcoholic Liver Diseases: a Meta-analysis // China Pharmacy. 2013; 24 (12): 1116-1118.7
Tekla GJ Van Rossum, Arnold G Vulto, Wim CJ Hop et al. Intravenous glycyrrhizin for the treatment of chronic hepatitis C: a double blind, randomized, placebo-controlled phase I/II trial // Journal of gastroenterology and hepatology. 1999; 14: 1093-1099.8
Drugs containing (Analogs)
Level 4 ATC code matches: Hepatosan
Sirepar
Hepatomax
Phosphogliv
Phosphogliv forte
Thiotriazolin
Karsil Forte
Darsil
Silibor
Karsil
Erbisol
Silimar
Methionine
Remaxol
Enerliv
Laennec
Progepar
Antral
Potassium orotate
Legalon
Trade name of Glycyrrhizic acid: Trisodium salt of glycyrrhizic acid, Epigen Intim, Glycyrrhizin.
The combination of Glycyrrhizic acid + Phospholipids is found in the preparations: Hepabos, Phosphogliv, Phosphogliv Forte .
Buy Phosphogliv capsules No. 50 in pharmacies
Trade name:
Phosphogliv®
International nonproprietary or generic name:
Glycyrrhizic acid + Phospholipids
Dosage form:
capsules.
Compound
for one capsule:
Active ingredients: phospholipids (Lipoid C 80) (calculated as 100% substance) (main component phosphatidylcholine 73-79%) - 65.0 mg, sodium glycyrrhizinate (trisodium salt of glycyrrhizic acid) - 35.0 mg.
Excipients: microcrystalline cellulose - 141.2 mg, calcium carbonate - 204.7 mg, calcium stearate - 0.9 mg, talc - 7.7 mg, colloidal silicon dioxide (aerosil) - 5.5 mg
Hard gelatin capsules - 96.0 mg [Capsule composition: body: sunset yellow dye (E110) - 1.0000%, titanium dioxide (E171) - 1.0000%, gelatin - up to 100.0%; cap: titanium dioxide (E171) - 0.2000%, iron dye black oxide (E172) - 3.5000%, gelatin - up to 100.0%].
Description:
hard gelatin capsules No. 0, capsule body – orange, cap – black. The contents of the capsule are granular powder from white with a slightly yellowish tint to light yellow, with a slight specific odor.
Pharmacotherapeutic group:
hepatoprotective agent
Pharmacological properties
Pharmacodynamics
Combined remedy. It has hepatoprotective, membrane-stabilizing and antiviral effects.
Glycyrrhizic acid has a hepatoprotective effect due to antioxidant, anti-inflammatory effects, as well as its effect on fibrogenesis.
Antioxidant action. Glycyrrhizic acid binds free oxygen radicals and inhibits enzymes that initiate lipid peroxidation (LPO) in hepatocytes.
Anti-inflammatory effect. Glycyrrhizic acid reduces inflammation due to its inhibitory effect on the NF-kB and TLR4 signaling pathways; inhibition of the production of proinflammatory cytokines (TNF α, IL-1, IL-6, IL-8); stimulating the production of anti-inflammatory cytokines (IL-2, IL-10, IL-12).
Glycyrrhizic acid inhibits 11β-hydroxysteroid dehydrogenase, which increases endogenous cortisol in the blood (pseudocorticosteroid effect).
The effect on fibrogenesis is associated with a decrease in type 1 collagen gene expression and a decrease in collagen production by hepatic stellate cells (Ito cells), as well as the destruction of activated Ito cells through the natural killer system, which helps slow the progression of fibrosis.
Suppresses the reproduction of viruses in the liver and other organs by stimulating the production of interferons, increasing phagocytosis, and increasing the activity of natural killer cells.
In case of skin lesions, due to its anti-inflammatory effect, it limits the spread of the process and promotes regression of the disease.
Phosphatidylcholine (the active substance of phospholipids) is the main structural element of cellular and intracellular membranes, restores their structure and function when damaged, providing a membrane-stabilizing effect. Normalizes protein and lipid metabolism, prevents the loss of enzymes and other active substances by hepatocytes, and helps restore liver function.
Pharmacokinetics
Glycyrrhizic acid
After oral administration in the intestine, under the influence of the enzyme β-glucuronidase, produced by bacteria of normal microflora, an active metabolite is formed from glycyrrhizic acid - β-glycyrrhetic acid, which is absorbed into the systemic circulation. In the blood, β-glycyrrhetic acid binds to albumin and is almost completely transported to the liver. The excretion of β-glycyrrhetic acid occurs predominantly in bile, with residual amounts in urine.
According to experimental data, phospholipids improve the lipophilic properties of glycyrrhizic acid, increasing the intensity and rate of its absorption by more than 2 times.
Phosphatidylcholine
More than 90% of phospholipids taken orally are absorbed in the small intestine. Most of them are cleaved by phospholipase A to 1-acetyl-lysophosphatidylcholine, 50% of which is reverse acetylated into polyunsaturated phosphatidylcholine during absorption in the intestinal mucosa. Polyunsaturated phosphatidylcholine enters the bloodstream through the lymph, from where it mainly enters the liver in the form of high-density lipoproteins. Pharmacokinetics in humans was studied using radiolabeled dilenoleyl phosphatidylcholine –3H (choline moiety) and 14C (linoleic acid moiety). The maximum concentration of 3H is achieved after 6-24 hours, accounting for 19.9% of the prescribed dose; 14C – after 4-12 hours, amounting to 27.9%. The half-life of the choline component is 66 hours, the linoleic acid residue is 32 hours. In feces, 2% 3H and 4.5% - 14C are detected; in urine – 6% 3H and a minimal amount of 14C. Both isotopes are absorbed in the intestine by more than 90%.
Indications for use
— Fatty liver degeneration (hepatosis), alcoholic, toxic, including medicinal, liver damage. — As part of complex therapy for viral hepatitis, liver cirrhosis and psoriasis.
Contraindications
- Hypersensitivity to glycyrrhizic acid, phosphatidylcholine or other components of the drug; — Antiphospholipid syndrome; — Pregnancy (data on effectiveness and safety are insufficient); — Breastfeeding period (data on effectiveness and safety are insufficient); — Children under 12 years of age (data on effectiveness and safety are insufficient).
Carefully
Use with caution in patients with portal hypertension and arterial hypertension. In patients with arterial hypertension. If you have these diseases, you should consult your doctor before starting to take the drug.
Use during pregnancy and breastfeeding
The drug is contraindicated during pregnancy and breastfeeding (there is insufficient data on effectiveness and safety).
Directions for use and doses
Take orally during meals, without chewing, with a small amount of liquid.
The recommended dosage regimen for adults and children over 12 years of age is 2 capsules 3 times a day. Duration of use can be up to 6 months, on average – 3 months.
Side effect
Usually the drug Phosphogliv is well tolerated, side effects develop very rarely, according to post-registration surveillance data - the frequency
Allergic reactions: skin rash, difficulty in nasal breathing, conjunctivitis, cough.
From the cardiovascular system: transient (transient) increase in blood pressure, peripheral edema.
From the digestive system: dyspepsia (belching, nausea, bloating), abdominal discomfort.
If these symptoms occur, you should stop taking the drug and consult your doctor.
Overdose
No cases of overdose have been reported.
Interaction with other drugs
Not described.
special instructions
If your blood pressure increases, you should stop taking the drug and consult a doctor.
Impact on the ability to drive vehicles and operate machinery
The drug does not have a negative effect on the ability to drive vehicles and perform other work that requires increased concentration and speed of psychomotor reactions.
Release form
Capsules.
30, 50, 100, 200 or 300 capsules in plastic containers with high-density polyethylene or propylene lids.
10 capsules per blister pack made of polyvinyl chloride film and aluminum foil.
1 container or 2, 3, 5 strip packaging along with instructions for use are placed in a cardboard pack.
Storage conditions.
Store at a temperature not exceeding 25 °C. Keep out of the reach of children.
Best before date.
3 years. After the expiration date, do not use the drug.
Vacation conditions.
Over the counter.