- Summary
- Introduction
- Vaccination
- Research results and discussion
- conclusions
- Literature
SUMMARY
To prevent seasonal acute respiratory viral infections, three main approaches are used: vaccination, the use of interferon inducers and interferon preparations. The epidemiological effectiveness of various prevention methods is discussed.
KG Gurevich Prevention of seasonal acute respiratory virus infections
For seasonal acute respiratory virus infections prophylactics the three common methods are used: vaccination, interferon-inductors drugs, and interferon. Epi-demiological effectiveness of different preventive methods is discussed.
INTRODUCTION
The problem of preventing acute respiratory viral infections (ARVI) currently seems very relevant due to the exceptionally high incidence rate. Surges in the number of people infected and sick with ARVI usually have the character of seasonal epidemics, which leads to significant economic losses. The high incidence of ARVI is associated with the exceptional ease of spread of viruses in crowded groups of people (at work, in transport, within the family, etc.) and the almost complete lack of opportunities to prevent the spread of viruses through the air. Persons from the so-called risk groups are most susceptible to the seasonal incidence of ARVI: children, the elderly, patients with various types of immunodeficiencies, bronchopulmonary diseases, kidney diseases, diabetes mellitus, cancer, hemophilia, etc. [18]. Currently, mainly symptomatic methods of treating ARVI have been developed; The exception is interferon preparations and inhibitors of virus entry into cells (rimantadine, arbidol). In 1960-80 There was a tendency towards the use of antibiotics (primarily the macrolide series) for the treatment of ARVI, which was not only unsuccessful, but also led to the emergence of a large number of resistant microorganisms [1]. Therefore, much attention is currently paid to the prevention of ARVI.
Prevention of ARVI can be carried out in three main areas: 1. Vaccination. 2. Use of immunomodulators and interferon inducers. 3. Use of interferon drugs.
Note that the latter direction of prevention can also be used for pathogenetically based treatment of ARVI.
Page 212
VACCINATION
Vaccination is carried out only against certain (previously known) strains of ARVI pathogens. Despite the fact that modern vaccines contain several strains of viruses, they are ineffective at the beginning of an epidemic, when the actual strains that caused the epidemic have not been identified. It also turns out to be ineffective if there is a mutation of the virus. In addition, in order for vaccination to be effective, it must be carried out 2-3 weeks before the outbreak of the epidemic. Significant limitations for vaccination are childhood (usually up to 7 years), pregnancy and lactation, hypersensitivity to vaccine components and immunodeficiencies [7]. Meanwhile, today vaccination is considered the most effective and safe method of preventing ARVI not only in humans, but also in animals [33]. Currently, both injection and intranasal types of vaccines have been developed. The effectiveness of the two types of vaccines is approximately equal and is more than 90% [32]. In many countries around the world, vaccination against seasonal epidemics is included in state medical programs [37]. It is interesting to note that vaccination not only has a passive effect on the immune system, as was previously believed, but actively stimulates the production of antibodies against the causative virus, apparently having a priming (preparatory) effect. Moreover, this effect is observed not only in healthy individuals, but also in people at risk [18]. It has been shown that preventive vaccination against ARVI is more justified from an economic point of view than subsequent treatment. Thus, as a result of a wide epidemiological study conducted in Finland, it was found that the cost of vaccination is 141 Finnish marks (FM) per person. The incidence among vaccinated people is approximately 5%, and among those who have not been vaccinated - 47%. If a person falls ill after vaccination, his treatment costs an average of 1,183 fm. If an unvaccinated person gets sick, then his treatment costs approximately 5.3 times more (6270 fm) [32]. The difference in treatment costs between vaccinated and unvaccinated individuals is likely due to the above-mentioned stimulation of the immune system under the influence of immunization. It has been shown that vaccination can reduce not only the likelihood of infection during an epidemic, but also the likelihood of cardiac complications in the event of infection [40], and reduce the number of deaths from ARVI [36].
IMMUNOMODULATION
Immunomodulation agents are most widely represented on the pharmacological market of the Russian Federation; existing drugs can be divided into three main groups: vitamins and microelements; interferon inducers; · other.
Preparations of vitamins and microelements have a general stimulating and immunomodulatory effect [5, 9]. In this case, both single preparations of vitamins and microelements and their complexes can be used. A double-blind, placebo-controlled trial showed that supplementation of zinc (10 mg per day as sulfate, 6 days per week for 6 months) in the diet of 100 children in Epiofia, aged 6 to 12 months, reduced the number of episodes of ARDB [42]. A multicentre study on the possible effect of selenium on the incidence of respiratory diseases in premature infants was conducted in New Zealand. 534 girls born weighing up to 1.5 kg were examined from the first week of life to the first menstruation. Selenium was given at a dose of 7 μg/kg/day parenterally in infancy or 5 mg/kg/day orally in older adults. An increase in the content of selenium and glutathione peroxidase in the blood plasma was described in the group taking selenium compared to the placebo group. Moreover, at an early age, low selenium levels were associated with an increased risk of death due to viral respiratory diseases [20]. A multicenter study conducted in the USA over 2 years showed that daily administration of even half the recommended dose of zinc and selenium to elderly people significantly reduces the incidence of acute respiratory viral infections [30]. The multicenter study SU.VI.MAX (SUPPlementation en Vitamines et Mineraux AntiOxidant Studi), conducted in France, included observation of 12,735 people for 8 years. Women aged 35-60 years and men aged 45-60 years were examined. Every day, the subjects received ME selenium - 100 mcg, zinc - 20 mg and antioxidant vitamins C - 120 mg, E - 30 mg, b-carotene - 6 mg (100-300% of the recommended dose). It was shown that the administration of microelements with vitamins reduced the risk of developing acute respiratory infections and acute respiratory viral infections [26]. A similar result was obtained in the multicenter study MIN.VIT.AOX [24]. It should be noted that in order for the immunomodulatory effects of microelements and vitamins to manifest themselves, their long-term use is necessary. Thus, in the SU.VI.MAX study, a significant decrease in the incidence of ARVI was observed only after 6 months. after taking vitamin-mineral complexes [23].
Interferon inducers.
Currently, double-blind, placebo-controlled trials have established the effectiveness of several small-molecule interferon inducers - amixin, cycloferon, bendazole and dipyridamole.
Moreover, the last two drugs initially had indications for use as vasodilators. Despite the fact that many vasodilators are potential interferon inducers [6], their clinical effectiveness has not been confirmed. Interferon inducers seem to be one of the most promising drugs for the prevention of seasonal epidemics of ARVI. This is due to the fact that they “turn on” natural antiviral defense mechanisms, which persist even after the use of these drugs [13]. Amiksin and cycloferon
are similar inducers of interferon biosynthesis, with the first being a predominant inducer of interferon-alfa/betta biosynthesis by T cells, and the second - interferon-alfa by B cells.
Double-blind, placebo-controlled trials have shown the clinical effectiveness of the drugs, which in the case of ARVI prevention reaches 80% [41]. Bendazole (dibazole, gliophene)
potentiates the formation of interferon [8].
Double-blind, placebo-controlled trials have shown that prophylactic use of bendazole is effective in more than 80% of cases for the prevention of seasonal epidemics of ARVI [10]. Currently, the Moscow government distributes Bendaloz to all schoolchildren of the city during epidemiological outbreaks of ARVI to prevent morbidity. Restrictions on the use of bendazole: old age, individual intolerance to the drug [7]. Dipyridamole (curantyl, persantyl, parsedyl, apodipyridamole)
is an interferon inducer both in vitro [2, 22] and in vivo [14, 15]. Placebo-controlled trials have shown that the drug is effective in more than 90% of cases for prevention during ARVI epidemics [11, 12]. Despite the fact that the effectiveness of the prophylactic use of dipyridamole is higher than that of bendazole, the drug has significant limitations for use: childhood (up to 12 years), pregnancy, breastfeeding due to the lack of data on the safety of use in these groups, individual intolerance to the drug [ 4, 7]. For a long time it was believed that dipyridamole could cause coronary steal syndrome in patients with coronary heart disease (CHD). It has now been proven that coronary steal can only develop with intravenous administration of the drug, and not parenteral, used for the prevention of ARVI [4]. However, caution is necessary when prescribing dipyridamole to persons with coronary artery disease.
Page 213
INTERFERON PREPARATIONS
The interferon system is the body's natural defense system. Its main role is to inhibit viral replication. Thus, the interferon system resists viral infections. However, in addition to the antiviral function, the interferon system also has a number of others: regulatory, neuromodulatory, etc. [6]. Quite often, suppression of interferon production is observed, leading to reduced resistance to viral infections and their frequent relapses. Even among the population of relatively healthy residents of Moscow, up to 20% have interferon production below normal. There are several reasons for the decrease in interferon biosynthesis: genetic (blood group II, Down syndrome) [3, 29], stress [14], lack of vitamins and/or microelements [5], etc. Prescribing interferon drugs in the case of reduced natural production of this cytokine plays a role the role of replacement therapy, which can be used both for the prevention and treatment of seasonal epidemics of ARVI, regardless of the strain of the virus that caused the epidemic [21, 38], which distinguishes interferon preparations from vaccines that are effective only against specific strains. Initially, human leukocyte interferon was used in clinical practice. However, the use of interferon obtained in human cell culture, even when using the most advanced control systems, cannot guarantee with 100% probability that the resulting drug does not contain human immunodeficiency viruses, hepatitis B, C, D, etc. In addition, despite the highly effective methods for cleaning the resulting raw materials, it is impossible to completely get rid of ballast proteins. Therefore, preparations of human leukocyte interferon are potentially strong allergens [6]. Meanwhile, interferon itself is not an allergen. Therefore, in connection with the development of genetic engineering, recombinant interferon preparations have been extremely widely used in recent years. Autoimmune diseases are a strict contraindication to the use of these drugs. During pregnancy and breastfeeding, recombinant interferons are used with caution [7]. It has been proven that when administered intranasally, interferons enter the blood and pass through the blood-brain barrier [6], that is, when administered intranasally, interferons have both local and central effects, which allows not only to mobilize the antiviral defense of the immune system, but also to activate the central nervous and endocrine systems so as to change metabolism along the path optimal for fighting viral infection [6, 13]. As a means of preventing ARVI, interferons are considered emergency measures. If it takes time for the effect of vaccination or immunomodulators to appear, then interferon preparations can be used immediately after contact with a patient with ARVI or at the first symptoms of the disease. Even if the first symptoms of ARVI appear, intranasal use of interferons allows one to avoid the manifestation of the disease in more than 80% of cases [19]. It was found that in children, prophylactic use of intranasal interferon preparations was more effective for the prevention of influenza than vaccination. At the same time, many children had contraindications to vaccination [31]. It was found that the greatest likelihood of contracting ARVI is within the family, with constant household contacts. Members of a family in which there was one person with ARVI were randomly (at the first sign of a cold) prescribed intranasal interferon or placebo 2 times a day for 2 days. It was shown that in the group receiving placebo, the number of people with clinical manifestations of ARVI remained more than 55%, while among those taking interferon it was less than 13% [27]. In a similar study conducted by Hayden FG et al. It has been shown that the effectiveness of using placebo to prevent ARVI is 39%, interferon is 79% [25]. Healthy volunteers were infected with rhinovirus. Patients were randomly assigned to receive interferon or placebo for 4 days. The use of interferon prevented the development of the disease in 90% of cases; placebo had no effect [28]. Four-week use of interferon during the epidemic reduced the incidence of acute respiratory viral infections by more than 75% compared to the group taking placebo [34]. Two-month prophylactic intranasal use of interferon (from the beginning of the ARVI epidemic) turned out to be effective in 76% of cases [35]. However, it should be noted that such long-term use of interferon drugs is undesirable, since the production of interferon in the body is controlled by a negative feedback mechanism [6]. Therefore, long-term administration of exogenous interferon leads to inhibition of the production of endogenous interferon. It has been shown that after a month of intranasal use of ARVI interferon, a person experiences an even more profound immunointerferon deficiency than before it began, which is accompanied by reduced resistance to viral infections [39]. Therefore, it is optimal to use interferon preparations for up to 5 days [6]. If necessary, the course can be repeated. In recent years, recombinant interferon-alfa2 preparations have appeared on the pharmacological market of the Russian Federation. Their preventive effectiveness is 87% [17], which is significantly higher than that of human leukocyte interferon. It has been shown that recombinant interferon-alfa2 is also effective for the treatment of ARVI: it shortens the time of clinical recovery of patients, reduces the severity of symptoms of the disease [16].
CONCLUSION
The currently widespread vaccine prevention of ARVI cannot provide 100% protection against seasonal epidemics of ARVI, which is due to the fact that there are more than 170 possible strains of the influenza virus, while the vaccine is usually effective against 3 strains. Therefore, drugs that affect the interferon system, which is a mechanism of natural antiviral defense, are increasingly being used to prevent ARVI. Both interferon inducers and interferon preparations are used. The advantage of these drugs is that they include natural defense mechanisms and have biological activity against all viruses that cause ARVI. From our point of view, these drugs are the most promising for the prevention of ARVI in the 21st century.
LITERATURE
1. Aleksanyan L.A., Vertkin A.L., Gurevich K.G., Ishchenko A.L., Kolobov S.V., Lobanova E.G., Pashkov K.A., Popkov S.A., Popkova A.M., Soldatenko I.V. Macrolides./ Ed. A.M. Popkova, A.L. Vertkina, S.V. Kolobova. - M.: Dialogue-MSU, 2000. - 108 p. 2. Galabov A.S., Masticova M. Dipyridamole - an interferon inducer. - Acta Virol., 1982. V. 26. P. 137-147. 3. Grigoryan S.S., Ershov F.I. The interferon system is normal and in pathology. - M., 1996. - P. 147-155. 4. Gurevich K.G., Lobanova E.G. Biochemical pharmacology of dipyridamole (Curantyl): mechanisms of action, clinical application. - Cardiology, 2000. T. 42. N12. pp. 87-91. 5. Gurevich K.G. Disorders of micronutrient metabolism and their correction. - Pharmateka, 2001. N3. pp. 45-53. 6. Ershov F.I. The interferon system is normal and pathological. - M.: Medicine, 1996. - 239 p. 7. Krylov Yu.F. Radar. Encyclopedia of drugs. M.: RLS-2001, 2001. - 1503 p. 8. Povolotsky I.L., Krivokhatskaya L.D. The influence of dibazole and ascorbic acid on the antiviral activity of human interferon in cell culture. - Antibiotics, 1989. T. 24. N4. pp. 291-294. 9. Podkolzin A.A., Dontsov V.I. Low intensity factors in bioactivation and immunocorrection. - M.: Panas-Aero, 1995. - 195 p. 10. Semenenko T.A., Perepelkin V.S., Prozorovsky S.V. Theoretical and preventive aspects of the prevention of infectious diseases. — Military. Honey. Zhurn., 1996. T. 317. © 8. P. 40-43. 11. Slepushkin A.N., Fedorova G.I., Kucherenko T.P., Biryukova I.G. On the tactics of using the immunomodulator “Curantila” for nonspecific prevention of acute respiratory diseases (ARI) at industrial enterprises. - in the book. “Viral infections”, Ekaterinburg, 1993. pp. 62-66. 12. Slepushkin A.N., Fedorova G.I. Clinical use of dipyridamole (Curantyl) for the prevention of acute respiratory diseases. - Wedge. Pharmacol. Ter., 2000. T. 9. N1. pp. 39-41. 13. Surkina I.D. Interferon-inducing effects of dipyridamole: antiviral and regulatory. - Ter. Archive, 2000. T. 72. N8. pp. 61-64. 14. Surkina I.D., Gotovtseva E.P., Gurevich K.G., Uchakin P.N. The use of dipyridamole (Curantyl) for the correction of stress-induced disorders of interferonogenesis and the prevention of infectious diseases. - Wedge. Pharmacology and therapy, 2000. T. 9. N2. pp. 39-43. 15. Surkina I.D., Gotovtseva E.P., Balashov A.M., Uchakin P.N., Gurevich K.G., Shkolnik N.M. Dipyridamole in the treatment of recurrent stress-induced opportunistic infectious diseases. - Russ. Honey. Journal, 2000. T. 8. N13-14. pp. 554-556. 16. Feliksova L., Shebekova V., Tselipanova E., Mikhailova N., Gaponyuk P. Grippferon in children with ARVI. - Doctor, 2001. N1. pp. 40-41. 17. Shumilov V.I., Shevtsov V.A., Lobov S.P. Influenza and ARVI: nonspecific prevention using genetically engineered alfa-2 interferon and its new forms. — Attending physician, 2000.N9. P.20-21. 18. Brydak LB, Machala M. Humoral immune response to influenza vaccination in patients from high risk groups. - Drugs, 2000. V. 60. N. 1. P. 35-53. 19. Cantell K. Development of antiviral therapy with alpha interferons: promises, false promises and achievements. -Ann. Med., 1995. V. 27. N. 1. P. 23-28. 20. Darlow BA, Winterborn CC, Inder TE, Graham PJ, Harding JE, Weston PJ, Austin NC, Elder DE, Mogridge N., Buss IH, Stuils KB The effect of selenium supplementation on outcome in very low birth weight infants: a randomized controlled trial. The New Zealand Neonatal Study Group. - J. Pediatr., 2000. V. 136. N. 4. P. 473-480. 21. Finter NB, Chapman S., Dowd P., Johnston JM, Manna V., Sarantis N., Sheron N., Scott G., Phua S., Tatum PB The use of interferon-alpha in virus infections. - Drugs, 1991. V. 42. N. 5. P. 749-765. 22. Galabov AS, Mastikova M. Dipyridamole induces interferon in man. - Biomed. Pharmacother., 1984. V. 38. P. 413-414. 23. Girodon F., Lombard M., Galan P., Brunet-Lecomte P., Monget AL, Arnaud J., Preziosi P., Hercberg S. Effect of micronutrient supplementation on infection in institutionalized elderly subjects: a controlled trial. —Ann. Nut. Methab., 1997. V. 41. N. 2. P. 98-107. 24. Girodon F., Galan P., Monget AL, Boutron-Runault MS, Brunet-Lecomte P., Preziozi P., Arnaud J., Manuguerra JC, Herchberg S. Im-pact of trace elements and vitamin supplementation on immunity and infection in institutionalized elderly patients: a randomized controlled trial. MIN.VIT.AOX. geriatric network. —Arch. Intern. Med., 1998. V. 159. N. 7. P. 748-754. [19] 25. Hayden FG, Albrecht JK, Kaiser DL, Gwaltney JM Jr. Prevention of natural colds by contact prophylaxis with intranasal alpha 2-interferon. — N. Engl. J. Med., 1986. V. 314. N. 2. P. 71-75. 26. Hercberg S., Galan P., Preziosi P., Roussel AM, Arnaud J, Richard MJ, Malvi D., Paul-Dauphin A., Briancon S., Favier A. Background and rationale behind the SU.VI.MAX study, a prevention trial using nutritional dose of a combination of antioxidant vitamins and minerals to reduce cardiovascular diseases and cancer. SUSupplementation et Min-eraux AntioXydants study. — Int J. Vitam. Nutr. Res., 1998. V. 68. N. 1. P. 3-20. 27. Herzong C., Berger R., Fernex M., Friesecke K., Havas L., Just M., Dubach UC Intranasal interferon (rIFN-alpha A, Ro 22-8181) for contact prophylaxis against common cold: a randomized , double-blind and placebo-controlled filed study. - Antiviral Res., 1986. V. 6. P. 171-176. 28. Higgins PG, Al-Nahib W., Wilman J., Tyrrell DA Interferon-beta ser as prophylaxis against experimental rhinovirus infection in volunteers. - J. Interferon Res., 1986. V.6. N. 2. P. 153-159. 29. Horisberger MA Interferones, Mx genes, and resistance to influenza virus. - Am. J. Resp. Crit. Care Med., 1995. V. 152. N. 4. Pt. 2. S67-S71. 30. Johnson MA, Porter KH Micronutrient supplementation and infection in institutionalized elders. - Nutr. Res., 1997. V. 55. N. 11. Pt 1. P. 400-404. 31. Kneyber MC, Moll HA, de Groot R. Treatment and prevention of respiratory virus infection. - Eur. J. Pediatr., 2000. V. 159. N. 6. P. 399-411. 32. Kumpulainen V., Makela M. Influenza vaccination among healthy employees: a cost-benefit analysis. — Scand. J. Infect. Dis., 1997. V. 29. N. 2. P. 181-185. 33. Lutticken D. Viral disease of the immune system and strategies to control infectious bursal disease by vaccination. — Acta Vet. Hung., 1997. V. 45. N. 3. P. 239-249. 34. Monto AS, Shope TC, Schwartz SA, Albrecht JK Intranasal interferon-alpha 2bretta for seasonal prophylaxis of respiratory infection. — J. Infect. Dis., 1986. V. 154. N. 1. P. 128-133. 35. Monto AS, Albrecht JK, Schwartz SA Demonstration of doseresponse relationship in seasonal prophylaxis of respiratory infections with alpha-2betta interferons. - Atimicrob. Agents Chemother., 1988. V. 32. N. 1. P. 47-50. 36. Monto AS The clinical efficacy of influenza vaccination. - Pharma-cogenomics., 1996. V. 9. Suppl. 3. P. 16-25. 37. Plotkin SA Vaccination against the major infectious diseases. - CR Acad. Sci. III, 1999. V. 322. N. 11. P. 943-951. 38. Saravolac EG, Sabula D., Crist C., Blasetti K., Schnell G., Yang H., Kande M., Levy HB, Wong JP Immunoprophylactic strategies against respiratory influenza virus infection. -Vaccine, 2001. V. 19. N. 17-19. P. 2227-2232. 39. Scott GM, Onwubalili JK, Robinson JA, Dore C, Secher DS, Cantell K. Tolerance of one-month intranasal interferon. — J. Med. Vi-rol., 1985. V. 17. N. 5. P. 99-106. 40. Siscovick DS, Raghunathan TE, Lin D., Weinmmann S., Arbogast P., Lemaitre RN, Psaty BM, Alexander R., Cobb LA Influenza vaccination and the risk of primary cardiac arrest. - Am. J. Epidemiol., 2000. V. 152. N. 7. P. 674-677. 41. Tazulakhova EB, Parshina OV, Guseva TS, Ershov FI Russian experience in screening, analysis, and clinical application of novel interferon inducers. - J. Interferon Cytokine Res., 2001. V. 21. N. 2. P. 65-73. 42. Umeta M., West CE, Haidar J., Derenberg P., Hutvast JG Zinc supplementation and stunted infants in Ethiopia: a randomized controlled trial. - Lancet, 2000. V. 355. P. 2021-2026.
MEDICAL CENTER
- Level of circulating interferon (serum interferon).
- Spontaneous production of interferon in vitro.
- Induced synthesis of alpha-interferon in vitro.
- Induced synthesis of interferon gamma in vitro.
Additional tests:
- Determination of sensitivity to interferon drugs (No. 1044 - Ingaron, No. 1045 - Intron, No. 1047 - Reaferon, No. 1048 - Realdiron, No. 1049 - Roferon).
- Determination of sensitivity to interferon inducers (No. 1050 - Amiksin, No. 1051 - Kagocel, No. 1052 - Neovir, No. 1053 - Ridostin, No. 1054 - Cycloferon).
- Determination of sensitivity to immunomodulators (No. 1055 - Galavit, No. 1056 - Gepon, No. 1057 - Immunal, No. 1058 - Imunofan, No. 1059 - Immunomax, No. 1060 - Likopid, No. 1061 - Polyoxidonium, No. 1062 - Taktivin, No. 1063 - Thymogen, No. 1066 - Imunorix, No. 1148 - Panavir, No. 1064 - Isoprinosine).
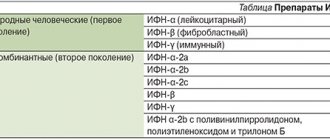
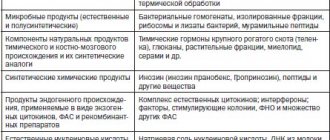
Interferons (IFNs) are the most important component of the body’s innate nonspecific defense against infections (the name interferons comes from their ability to interfere with viral infection of cells).
This is a family of proteins of local (autocrine and paracrine) regulation that are capable of activating intracellular processes and intercellular interactions that provide resistance to viral infections, enhance innate and acquired immune responses, modulate the processes of development and death of normal and tumor cells. The body's resistance to viral infections and a number of other diseases largely depends on the activity of a group of genes of the interferon system. Interferon preparations are widely used in medicine. The effects of interferons are indirect - activation of specific receptors by interferons causes a cascade of cellular processes leading to the induction of specific interferon-stimulated genes encoding the synthesis of many proteins, which provide the antiviral effects, antitumor and antiproliferative effects of interferons. Proteins induced by interferons include: enzymes, transcription factors, cell surface glycoproteins, cytokines, chemokines and other factors, the effect of which continues to be studied. The production of interferons by cells is transient, temporary - normally “silent” interferon genes are induced under the influence of products of viral and microbial origin and chemical inducers.
Interferons are divided into three types (α, β and γ), which are associated with specific functions and specific producing cells. Interferons α and β, despite significant structural differences, have common receptors and similar functions. Together they are also called type I interferons, or acid-stable interferons, in contrast to interferon-γ, which has its own receptors and partially different functions (it is also known as interferon II, or acid-labile interferon).
Interferon α (more than 20 of its subtypes have been identified) is the main interferon that is synthesized in a virus-induced leukocyte culture. The main producers of IFN-α are plasmacytoid dendritic cells; monocytes make a significant contribution to the IFN-α-producing ability of the blood. Its main functions are antiviral activity and activation of natural killer cells.
Interferon β is the main interferon produced by double-stranded RNA-induced fibroblast culture. Its main producers are fibroblasts, epithelial cells and macrophages; its main function is antiviral activity.
Interferon γ is the main interferon produced by immunologically stimulated (mitogens or antigens) lymphocyte cultures. The main producing cells of IFN-γ are T-lymphocytes. The main function of interferon gamma is immunoregulation (including activation of macrophages, enhancement of the Th1 response, induction of the expression of major histocompatibility complex type II antigens on antigen-presenting cells, etc.); like other interferons, it exhibits antiviral and antiproliferative activity. All animal cells are capable of producing interferons; certain cells (leukocytes and fibroblasts) can produce more than one type - both IFN-α and IFN-β.
Studying the parameters of interferon status allows us to identify insufficiency of the interferon system. Assessment of detected changes can serve as a guide in the diagnosis, treatment and prognosis of diseases of both viral and non-viral etiology. Healthy people are characterized by low levels of serum interferon and high values of induced interferon synthesis. Stress and acute viral infections, allergic conditions are accompanied by an increase in the level of circulating interferon and a decrease in the level of induced production of alpha and gamma interferons by leukocytes. In bronchial asthma and urticaria, the level of circulating interferon correlates with the severity of the disease.
Chronic viral infections (herpes, hepatitis), multiple sclerosis are accompanied by suppression of all indicators of interferon status. Autoimmune diseases (systemic lupus erythematosus, rheumatoid arthritis) are characterized by suppression of inducible production of interferon alpha. Acute lymphocytic leukemia and malignant formations are accompanied by suppression of induced interferon gamma production. The results of the study of interferon status should be considered in conjunction with other laboratory and clinical anamnestic data. A decrease in the production of alpha and gamma interferon, which can be both a cause and a consequence of acute and chronic viral diseases, indicates a congenital or acquired deficiency of the interferon system and can be considered as an indication for interferon-stimulating therapy. Normalization of interferon status indicators usually coincides with the recovery process. In people over 50 years of age, insufficiency of the interferon system is relatively more common. The study of interferon status parameters with determination of sensitivity to drugs is used to select effective therapy when using exogenous interferon drugs, interferon inducers and immunomodulators.
Interferon preparations
- Ingaron is a recombinant human interferon-γ.
- Intron - recombinant human interferon-α-2b.
- Reaferon is a recombinant human interferon-α-2.
- Realdiron is a recombinant human interferon-α-2b.
- Roferon is a recombinant human interferon-α-2a.
Interferon inducers
- Amiksin (international non-patent name - tilorone): dihydrochloride 2,7-bis-[2(diethylamino)-ethoxy]-fluorene-9-OH-dihydrochloride.
- Kagocel: active substance - sodium salt of the copolymer (1-4)-6-0-carboxymethyl-bD-glucose, (1-4)-bD-glucose, (21-24)-2,3,14,15,21, 24,29,32-octahydroxy-23-(carboxy-methoxymethyl)-7,10-dimethyl-4,13-di(2-propyl)-19,22,26,30,31-pentaoxaheptacyclo [23.3.2.216.05.28 .08.27.09.1В.012.17] dotriaconta-1,3,5(28),6,8(27),9(18),10,12(17),13,1-decaene.
- Neovir – 2-(9-oxo-9,10-dihydroacridin-10-yl) sodium acetate.
- Ridostin is a mixture of sodium salts of double-stranded and single-stranded RNA.
- Cycloferon – meglumine acridone acetate.
Immunomodulators
- Galavit is a phthalhydrazide derivative.
- Hepon is a synthetic peptide consisting of 14 amino acid residues.
- Immunal is a preparation of Echinacea purpurea juice.
- Immunofan is a hexapeptide (arginyl-alpha-aspartyl-lysyl-valyl-tyrosyl-arginine).
- Immunomax is an acidic peptidoglycan with a molecular weight of 1,000 - 40,000 kDa.
- Lykopid - active substance - glucosaminylmuramyl dipeptide - 4-O-(2-acetylamino-2-deoxy-beta-D-glucopyranosyl)-N-acetylmuramyl]-L-alanyl-D-alpha-glutalamide.
- Polyoxidonium - international non-proprietary name / composition: Azoximer (Azoximer) - N-oxidized derivative of polyethylene piperazine.
- Taktivin is a complex of polypeptides from the thymus gland of cattle.
- Thymogen is a polypeptide from the thymus gland of cattle.
- Panavir is a purified extract of shoots of the Solanum tuberosum plant, the main active ingredient is a hexose glycoside consisting of glucose, rhamnose, arabinose, mannose, xylose, galactose, uronic acids.
ANTIVIRAL DRUGS WITH AN EXTENDED SPECTRUM OF ACTIVITY
MODERN ANTIMICROBIAL CHEMOTHERAPY
MODERN ANTIMICROBIAL CHEMOTHERAPY
L.S. Strachunsky, S.N. Kozlov. Guide for doctors
| Content | ANTIBIOTIC.ru |
Antiviral drugs
RIBAVIRIN
Virazol, Rebetol
It has a wide spectrum of activity against many DNA and RNA viruses and is highly toxic. The mechanism of antiviral action is not fully understood.
Activity spectrum
Of clinical importance is activity against respiratory syncytial virus, as well as viruses that cause Lassa fever, hemorrhagic fever with renal syndrome and hepatitis C (in combination with interferon-alpha).
Pharmacokinetics
Bioavailability when taken orally is 35-45%. When administered by inhalation, high concentrations are observed in respiratory secretions and significantly lower concentrations in plasma. With repeated administrations it can accumulate in red blood cells. Penetrates through the BBB. Metabolized in the liver, excreted in the urine. T1/2 - 30-60 hours, increases with renal failure.
Adverse reactions
- Local - rash, irritation of the skin, mucous membranes of the eyes and respiratory tract, bronchospasm (noted by both patients and medical staff
when using an aerosol dosage form). Ribavirin may crystallize in the respiratory tract and endotracheal tubes. - Hematotoxicity - anemia, lymphocytopenia (in AIDS patients); hemolytic anemia (usually by 4 weeks), reversible, does not require specific treatment, normalization of hemoglobin occurs with a temporary dose reduction.
- Neurotoxicity - headaches, fatigue, irritability, insomnia.
- Gastrointestinal tract - metallic taste in the mouth, abdominal pain, flatulence, nausea.
- Teratogenic effect.
Indications
- Infections caused by RSV (serologically confirmed): severe bronchiolitis and pneumonia in newborns and young children at risk (congenital heart defects, immunodeficiency, bronchopulmonary dysplasia), as well as those associated with severe cystic fibrosis or pulmonary hypertension.
- Lassa fever.
- Hemorrhagic fever with renal syndrome.
- Hepatitis C (in combination with interferon-alpha or peginterferon alfa).
Contraindications
Absolute
- Pregnancy.
- End-stage renal failure.
- Anemia.
- Hemoglobinopathies.
- Severe heart failure.
Relative
- Uncontrolled hypertension.
- Elderly age.
Warning
Due to its teratogenic effects, it is contraindicated during pregnancy and poses a danger to medical staff if pregnant.
All women receiving ribavirin (and if their partners are receiving it) should be protected against pregnancy during the entire course of therapy and for 4 months after the end of treatment. The pregnancy test must be repeated monthly, as well as for 4 months after the end of treatment. If pregnancy occurs during this period, the patient must be warned about the high risk of teratogenic effects.
In order to “protect” medical staff, inhaled administration of ribavirin is allowed only with the use of a special nebulizer.
Before using ribavirin, mandatory serological confirmation of the presence of RSV infection is required, as well as determination of HCV RNA by polymerase chain reaction (in patients with hepatitis C).
Dosage
Adults
For Lassa fever and hemorrhagic fever: intravenously - the first dose is 2.0 g, then 1.0 g every 6 hours for 4 days and then 0.5 g every 8 hours for 6 days.
For hepatitis C: orally 1.0-1.2 g per day for 12 months.
Newborns and children
For RSV infection: inhalation (using a nebulizer) 20 mg/ml (6.0 g in 300 ml of sterile water) for 18 hours a day, course of treatment - 3-7 days.
Release form
Bottles of 6.0 g of powder for preparing a solution for infusion; capsules 0.2 g.
LAMIVUDINE
Zeffix, Epivir TriTC
Active against retroviruses and hepatitis B virus.
Pharmacodynamics
In cells affected by the virus, it is activated, turning into lamivudine triphosphate, which inhibits hepatitis B virus DNA polymerase and HIV reverse transcriptase. There have been cases of development of hepatitis B virus resistance.
Pharmacokinetics
Bioavailability when taken orally is 86-88%. Distributed into many tissues and secretions, passes through the BBB. Excreted by the kidneys. T1/2 - 5-7 hours, with renal failure it may be extended.
Adverse reactions
Generally well tolerated. In rare cases, it causes lactic acidosis and hepatomegaly with steatosis, which may be associated with impaired mitochondrial function.
Indications
- Chronic hepatitis B.
Warning
With monotherapy, resistance to lamivudine of both the hepatitis B virus and HIV can develop quite quickly if double infection occurs.
Dosage
Adults
Orally - 0.1 g once a day for a year; in HIV-infected patients - 0.15 g every 12 hours.
Release form
Tablets 0.1 g.
Interferons are a group of biologically active proteins synthesized by the cell during the protective reaction. Interferon is secreted into the extracellular fluid and acts on other cells through receptors, increasing resistance to intracellular microorganisms, primarily viruses. Interferons do not have specificity and inhibit the replication of various viruses. The main mechanism of the antiviral action of interferon is to suppress the synthesis of viral proteins.
According to their structure and biological properties, interferons are divided into three types: α, β, γ
. According to the method of production, leukocyte, lymphoblastoid and recombinant interferons are distinguished. Recombinant alpha interferons are most widely used as antiviral drugs. In recent years, pegylated interferons (peginterferons) have been developed, obtained by adding polyethylene glycol and having a higher half-life and clinical efficacy.
INTERFERON-ALPHA: GENERAL PROPERTIES
Pharmacokinetics
Being a protein, interferon-alpha is destroyed in the gastrointestinal tract, therefore it is used only parenterally. When administered intramuscularly, the bioavailability is 80%, the maximum concentration in the blood is achieved on average after 3.8 hours. Low concentrations have been noted in the secretions of the respiratory tract, eye tissues, and the central nervous system. It is rapidly inactivated in the kidneys, and to a lesser extent in the liver. T1/2 - 2-4 hours, does not change with renal failure. Peginterferon alfa has a longer T1/2.
Adverse reactions
Adverse reactions of recombinant interferon-alpha are dose-dependent.
Early
(usually in the first week of treatment).
- The flu-like syndrome, manifested by fever, myalgia, and soreness of the eyeballs, usually disappears after 4-5 injections and does not require dose reduction or discontinuation of the drug.
Preventive measures:
prescribing paracetamol before administering interferon.
Late
(at 2-6 weeks of therapy, often causes interferon withdrawal).
- Hematotoxicity - anemia, thrombocytopenia, agranulocytosis.
- Neurotoxicity - drowsiness, lethargy, depression, less often convulsions.
- Cardiotoxicity - arrhythmias, transient cardiomyopathy, arterial hypotension.
- Autoimmune thyroiditis.
- Hyperlipidemia.
- Alopecia, skin rashes. Control measures:
control of hematopoiesis, levels of liver enzymes, electrolytes, ECG.
Drug interactions
Interferon alpha inhibits microsomal liver enzymes (cytochrome P-450), and therefore can disrupt the metabolism of many drugs (theophylline, etc.), increasing their concentration in the blood.
Due to the risk of adverse reactions from the central nervous system, narcotic, hypnotic and sedative drugs should be used simultaneously with interferon alpha.
Indications
- Chronic hepatitis B (in the presence of viral replication: HBV, DNA, HBeAg, in blood serum) and elevated levels of transaminases.
- Acute hepatitis C.
- Chronic hepatitis C (HCV RNA in blood serum), elevated levels of transaminases.
Contraindications
Absolute
- Psychosis (at the time of treatment or in history).
- Severe depression.
- Neutropenia or thrombocytopenia.
- Severe heart pathology.
- Decompensated cirrhosis of the liver.
- Uncontrollable seizures.
- Organ transplantation (except liver).
Relative
- Autoimmune diseases.
- Uncontrolled diabetes.
Dosage
Adults
Chronic hepatitis B
5 million IU daily or 10 million IU 3 times a week for 4-6 months.
Acute hepatitis C
High dose regimen - 10 million IU daily until transaminases normalize, then 3 million IU 3 times a week for 6 months.
Medium dose regimen: 5 million IU 3 times a week for 2 months, then 3 million IU 3 times a week for 4-10 months.
If tolerance is poor, switch to a low dose regimen of 3 million IU 3 times a week for 3-6 months.
Chronic hepatitis C
Monotherapy - 3 million IU 3 times a week for 12 months. In the absence of a response (preservation of HCV RNA in the blood serum), combination therapy is advisable after 3 months from the start of therapy.
Combination therapy:
- 1) Interferon alpha + ribavirin. For body weight ≤75 kg, interferon alpha 3 million IU 3 times a week, ribavirin 1 g/day (2 capsules in the morning + 3 capsules in the evening). For body weight >75 kg, interferon alpha 3 million IU 3 times a week, ribavirin 1.2 g/day (3 capsules in the morning + 3 capsules in the evening).
2) Peginterferon alfa-2b + ribavirin. For body weight <65 kg, peginterferon alfa-2b 1.5 gc/kg once a week, ribavirin 0.8 g/day. For a body weight of 65-85 kg, peginterferon alfa-2b 1.5 g/kg once a week, ribavirin 1 g/day. For body weight >85 kg, peginterferon alfa-2b 1.5 mg/kg once a week, ribavirn 1.2 g/day.
For chronic hepatitis C - 3 million IU 3 times a week for 3 months, with normalization of transaminase levels and a decrease in the concentration of HCV RNA - administration at the same dose for 9-15 months. If, after 3 months from the start of therapy, the ALT level remains elevated and HCV RNA continues to be detected, the dose can be increased to 5 million IU 3 times a week.
Children over 1 year old
The effectiveness and safety of interferons in children have not been fully established. Controlled studies completed to date have revealed the effectiveness of the following treatment regimens: chronic hepatitis B - 6 million IU/m2 body surface 3 times a week for 6 months; chronic hepatitis C - 3-5 million IU/m2 3 times a week for 12 months.
INTERFERON ALPHA PREPARATIONS
Recombinant interferons
All commercial drugs in this group are a recombinant form of human α2-interferon, so their pharmacological action is similar. Depending on the amino acid content, interferon alpha-2a and interferon alpha-2b are distinguished, which do not differ significantly in clinical effectiveness and safety.
INTERFERON ALPHA-2a
Roferon-A, Reaferon
Release forms
Vials and ampoules of 3, 9 and 18 million IU ( Roferon-A
) and ampoules of 1 million IU (
Reaferon
) powder for the preparation of a solution for injection.
INTERFERON ALPHA-2b
Intron-A, Realdiron
Release forms
Bottles of 1, 3, 5 and 10 million IU ( Intron-A
) and ampoules of 1, 3 and 6 million IU (
Realdiron
) powder for the preparation of solution for injection.
PEGINTERFERON ALPHA-2b
PegIntron
A compound of interferon alfa-2b with polyethylene glycol (PEGylated interferon alfa-2b). It has a prolonged effect and higher therapeutic activity. Prescribed once a week. Recommended for the treatment of hepatitis C in persons who have contraindications to ribavirin, as well as in case of discontinuation of ribavirin due to the development of anemia.
Release forms
Vials of 50, 80 and 100 mcg of powder for the preparation of solution for injection.
| Copyright © 2000-2007 ANTIBIOTIC.ru Posted: 05/15/2004 |
The address of this page: https://www.antibiotic.ru/books/mach/mac0403.shtml
Last modified date: 05/24/2004 18:56