Nosological classification (ICD-10)
- A41.9 Septicemia, unspecified - A49.3 Infection caused by mycoplasma, unspecified - A59.0 Urogenital trichomoniasis - A74.9 Chlamydial infection, unspecified - B00.0 Herpetic eczema - B00.1 Herpetic vesicular dermatitis - B18 Chronic viral hepatitis - B25. 9 Cytomegalovirus disease, unspecified - B34 Viral infection of unspecified localization - B37.3 Candidiasis of the vulva and vagina (N77.1) - G03.9 Meningitis, unspecified - J06.9 Acute upper respiratory tract infection, unspecified - J11 Influenza, virus not identified - J18 Pneumonia, unspecified clarification of the pathogen - N39.0 Urinary tract infection without established localization - N76 Other inflammatory diseases of the vagina and vulva - O35.3 Damage to the fetus (suspected) as a result of a viral disease of the mother, requiring medical care for the mother - O35.8 Other anomalies and lesions of the fetus ( suspected) requiring provision of medical care to the mother
Composition and release form
Ointment for external and local use 1 g
- recombinant human interferon alpha-2 40000 IU - tocopherol acetate 0.002 g - excipients: anhydrous lanolin;
medical Vaseline; peach oil; purified water in aluminum tubes of 12 g; 1 tube in a cardboard box. Gel for local use 1 ml
- recombinant human interferon alpha-2 36000 IU - excipients: alpha-tocopherol acetate solution - 5%; methionine alcohol solution - 2%; benzoic acid solution - 0.4%; citric acid - solution - 10%; sodium tetraborate solution - 3%; sodium chloride solution - 10%; human serum albumin solution - 10%; glycerin - distilled; sodium carboxymethylcellulose; purified water - in aluminum tubes of 10 ml; 1 tube in a cardboard pack
Suppositories for rectal use 1 sup.
- human recombinant interferon alpha-2: - 150,000 IU - 500,000 IU - 1,000,000 IU - 3,000,000 IU - excipients: ascorbic acid - 0.015 g (150,000 IU), 0.022 g (500,000 IU, 1,000,000 IU, 3,000,000 IU ); tocopherol acetate - 0.055 g; base - cocoa butter or solid fat in PVC/PVC strip packaging of 10 pcs.; in a cardboard pack 1 package.
Clinical pharmacology
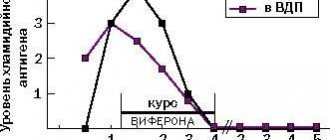
Biological properties: Human recombinant interferon alpha-2 has pronounced antiviral, immunomodulatory and antiproliferative properties. The complex composition of the drugs (ointment, suppository, gel) causes a number of new additional effects: in the presence of antioxidants (tocopherol acetate and/or ascorbic acid, benzoic or citric acids, as well as methionine), the specific antiviral activity of human recombinant alpha-2 interferon increases, its immunomodulatory effect on T- and B-lymphocytes, the level of immunoglobulin E is normalized, and the functioning of the endogenous interferon system is restored. Alpha-tocopherol acetate, ascorbic acid, benzoic and citric acids, as well as methionine, being highly active antioxidants, have pronounced anti-inflammatory, membrane-stabilizing and regenerating properties. It has been established that when using the drug Viferon rectal suppositories, there are no side effects that occur with parenteral administration of interferon preparations, and antibodies that neutralize the antiviral activity of interferon are not formed.
Viferon
Description of the drug VIFERON® (VIFERON) Based on the instructions for use of the drug officially approved in 2021, update date: 08.2015.11
Release form, packaging and composition Clinical-pharmacological. group Pharmacotherapeutic group Pharmacological action Indications of the drug Dosage regimen Side effects Contraindications for use Special instructions Overdose Drug interactions Dispensing conditions from pharmacies Storage conditions Expiration date Contacts for requests
Registration certificate holder: FERON, LLC (Russia)
Contacts for inquiries: FERON LLC (Russia) ATX code: L03AB05 (Interferon alfa-2b) Active substance: interferon alfa-2b (interferon alfa-2b) USAN accepted for use in the USA
Dosage forms
Viferon®
Rectal suppositoriesreg. No.: P N000017/01 from 06.10.10 - Indefinitely
Release form, packaging and composition of Viferon®
Rectal suppositories are white with a yellowish tint, bullet-shaped, uniform in consistency, heterogeneity of color in the form of marbling and the presence of a funnel-shaped depression on the longitudinal section are allowed, the diameter of the suppository is no more than 10 mm.
1 sup.
Interferon alpha-2b human recombinant 150,000 IU
Excipients: α-tocopherol acetate - 55 mg, ascorbic acid - 5.4 mg, sodium ascorbate - 10.8 mg, disodium edetate dihydrate - 100 mcg, polysorbate 80 - 100 mcg, cocoa butter base and confectionery fat - up to 1 g.
10 pieces. — cellular contour packages (1) — cardboard packs.
Rectal suppositories are white with a yellowish tint, bullet-shaped, uniform in consistency, heterogeneity of color in the form of marbling and the presence of a funnel-shaped depression on the longitudinal section are allowed, the diameter of the suppository is no more than 10 mm.
1 sup.
Interferon alpha-2b human recombinant 500,000 IU
-“- 1,000,000 IU
-“- 3,000,000 IU
Excipients: α-tocopherol acetate - 55 mg, ascorbic acid - 8.1 mg, sodium ascorbate - 16.2 mg, disodium edetate dihydrate - 100 mcg, polysorbate 80 - 100 mcg, cocoa butter base and confectionery fat - up to 1 g.
10 pieces. — cellular contour packages (1) — cardboard packs.
Clinical and pharmacological group: Interferon. Immunomodulatory drug with antiviral effect Pharmacotherapeutic group: MIBP-cytokine
pharmachologic effect
Human recombinant interferon alpha-2b has antiviral, immunomodulatory, antiproliferative properties, suppresses the replication of RNA and DNA viruses. The immunomodulatory properties of interferon alpha-2b, such as increased phagocytic activity of macrophages, increased specific cytotoxicity of lymphocytes to target cells, determine its indirect antibacterial activity.
In the presence of ascorbic acid and alpha-tocopherol acetate, the specific antiviral activity of interferon alpha-2b increases, its immunomodulatory effect is enhanced, which makes it possible to increase the effectiveness of the body's own immune response to pathogenic microorganisms. When using the drug, the level of secretory immunoglobulins of class A increases, the level of immunoglobulin E normalizes, and the functioning of the endogenous interferon alpha-2b system is restored. Ascorbic acid and alpha-tocopherol acetate, being highly active antioxidants, have anti-inflammatory, membrane stabilizing, and regenerating properties. It has been established that when using the drug Viferon® there are no side effects that occur with parenteral administration of interferon alfa-2b preparations, and no antibodies are formed that neutralize the antiviral activity of interferon alfa-2b. The use of the drug Viferon® as part of complex therapy makes it possible to reduce therapeutic doses of antibacterial and hormonal drugs, as well as reduce the toxic effects of this therapy.
Cocoa butter contains phospholipids, which make it possible not to use synthetic toxic emulsifiers in production, and the presence of polyunsaturated fatty acids facilitates the administration and dissolution of the drug.
Indications for the drug Viferon®
acute respiratory viral infections, including influenza, incl. complicated by a bacterial infection, pneumonia (bacterial, viral, chlamydial) in children and adults as part of complex therapy, infectious and inflammatory diseases of newborns, incl. premature babies, such as meningitis (bacterial, viral), sepsis, intrauterine infection (chlamydia, herpes, cytomegalovirus infection, enterovirus infection, candidiasis, including visceral, mycoplasmosis), as part of complex therapy, chronic viral hepatitis B, C, D in children and adults as part of complex therapy, incl. in combination with the use of plasmapheresis and hemosorption for chronic viral hepatitis of pronounced activity, complicated by cirrhosis of the liver, infectious and inflammatory diseases of the urogenital tract (chlamydia, cytomegalovirus infection, ureaplasmosis, trichomoniasis, gardnerellosis, human papillomavirus infection, bacterial vaginosis, recurrent vaginal candidiasis, mycoplasmosis) in adults as part of complex therapy, primary or recurrent herpetic infection of the skin and mucous membranes, localized form, mild and moderate course, incl. urogenital form in adults. ICD-10 codes
Dosage regimen
The drug is administered rectally.
1 suppository contains human recombinant interferon alpha-2b as an active substance in the indicated dosages (150,000 IU, 500,000 IU, 1,000,000 IU, 3,000,000 IU).
Acute respiratory viral infections, including influenza, incl. complicated by bacterial infection, pneumonia (bacterial, viral, chlamydial) in children and adults as part of complex therapy
The recommended dose for adults, including pregnant women and children over 7 years of age is Viferon® 500,000 IU, 1 suppository 2 times a day every 12 hours every day for 5 days. According to clinical indications, therapy can be continued.
Children under 7 years old, incl. newborns and premature infants with a gestational age of more than 34 weeks are prescribed Viferon® 150,000 IU, 1 suppository 2 times a day every 12 hours every day for 5 days. According to clinical indications, therapy can be continued. The break between courses is 5 days.
Premature newborns with a gestational age of less than 34 weeks are prescribed Viferon® 150,000 IU, 1 suppository 3 times a day after 8 hours every day for 5 days. According to clinical indications, therapy can be continued. The break between courses is 5 days.
Infectious and inflammatory diseases of newborns, incl. premature babies, such as meningitis (bacterial, viral), sepsis, intrauterine infection (chlamydia, herpes, cytomegalovirus infection, enterovirus infection, candidiasis, including visceral, mycoplasmosis) as part of complex therapy
Recommended dose for newborns, incl. premature babies with a gestational age of more than 34 weeks - Viferon® 150,000 IU daily, 1 suppository 2 times a day after 12 hours. The course of treatment is 5 days.
Premature newborns with a gestational age of less than 34 weeks are prescribed Viferon® 150,000 IU daily, 1 suppository 3 times a day every 8 hours. The course of treatment is 5 days.
Recommended number of courses for various infectious and inflammatory diseases: sepsis - 2-3 courses, meningitis - 1-2 courses, herpes infection - 2 courses, enterovirus infection - 1-2 courses, cytomegalovirus infection - 2-3 courses, mycoplasmosis, candidiasis, incl. visceral - 2-3 courses. The break between courses is 5 days. According to clinical indications, therapy can be continued.
Chronic viral hepatitis B, C, D in children and adults as part of complex therapy, incl. in combination with the use of plasmapheresis and hemosorption for chronic viral hepatitis of pronounced activity, complicated by cirrhosis of the liver
The recommended dose for adults is Viferon® 3,000,000 IU, 1 suppository 2 times a day every 12 hours every day for 10 days, then three times a week every other day for 6-12 months. The duration of treatment is determined by clinical effectiveness and laboratory parameters.
For children under 6 months of age, 300,000-500,000 IU/day is recommended, for children aged 6 to 12 months - 500,000 IU/day.
For children aged 1 to 7 years, 3,000,000 IU per 1 m2 of body surface area/day is recommended.
For children over 7 years old, 5,000,000 IU per 1 m2 of body surface area/day is recommended.
The drug is used 2 times a day after 12 hours for the first 10 days daily, then three times a week every other day for 6-12 months. The duration of treatment is determined by clinical effectiveness and laboratory parameters.
The daily dose of the drug for each patient is calculated by multiplying the recommended dose for a given age by the body surface area calculated using the nomogram for calculating body surface area by height and weight according to Garford, Terry and Rourke. The calculation of a single dose is carried out by dividing the calculated daily dose into 2 administrations, the resulting value is rounded up to the dosage of the suppository.
In case of chronic viral hepatitis of pronounced activity and liver cirrhosis, before plasmapheresis and/or hemosorption, children under 7 years of age are prescribed Viferon® 150,000 IU, children over 7 years of age - Viferon® 500,000 IU, 1 suppository 2 times a day after 12 h daily for 14 days.
Infectious and inflammatory diseases of the urogenital tract (chlamydia, cytomegalovirus infection, ureaplasmosis, trichomoniasis, gardnerellosis, human papillomavirus infection, bacterial vaginosis, recurrent vaginal candidiasis, mycoplasmosis) in adults, including pregnant women as part of complex therapy
The recommended dose for adults is Viferon® 500,000 IU, 1 suppository 2 times a day every 12 hours every day for 5-10 days. According to clinical indications, therapy can be continued.
Pregnant women from the second trimester of pregnancy (starting from the 14th week of gestation) are prescribed Viferon® 500,000 IU, 1 suppository 2 times a day every 12 hours every day for 10 days, then 1 suppository 2 times a day every 12 hours every fourth day for 10 days. Then every 4 weeks until delivery - Viferon® 150,000 IU, 1 suppository 2 times a day after 12 hours every day for 5 days. If necessary, Viferon® 500,000 ME is prescribed before delivery (from the 38th week of gestation), 1 suppository 2 times a day after 12 hours every day for 10 days.
Primary or recurrent herpetic infection of the skin and mucous membranes, localized form, mild to moderate course, incl. urogenital form in adults, including pregnant women
The recommended dose for adults is Viferon® 1,000,000 IU, 1 suppository 2 times a day every 12 hours every day for 10 days or more for recurrent infections. According to clinical indications, therapy can be continued. It is recommended to begin treatment immediately when the first signs of damage to the skin and mucous membranes appear (itching, burning, redness). When treating recurrent herpes, it is advisable to begin treatment in the prodromal period or at the very beginning of signs of relapse.
Pregnant women from the second trimester of pregnancy (starting from the 14th week of gestation) are prescribed Viferon® 500,000 IU, 1 suppository 2 times a day every 12 hours every day for 10 days, then 1 suppository 2 times a day every 12 hours every fourth day for 10 days. Then every 4 weeks until delivery - Viferon® 150,000 IU, 1 suppository 2 times a day after 12 hours every day for 5 days. If necessary, it is indicated before delivery (from the 38th week of gestation) Viferon® 500,000 IU, 1 suppository 2 times a day after 12 hours every day for 10 days.
Side effect
Allergic reactions: rarely - skin rash, itching. These phenomena are reversible and disappear 72 hours after stopping the drug.
Contraindications for use: hypersensitivity to any of the components of the drug.
Use during pregnancy and breastfeeding
The drug is approved for use from the 14th week of pregnancy.
There are no restrictions for use during lactation.
Use in children
Can be used in children according to indications.
special instructions
Impact on the ability to drive vehicles and operate machinery
Not installed.
Overdose Data on overdose of the drug Viferon® are not provided.
Drug interactions
Viferon® is compatible and goes well with all medications used in the treatment of the above diseases (including antibiotics, chemotherapy, corticosteroids).
Storage conditions Viferon®
The drug should be stored out of the reach of children, protected from light at a temperature of 2° to 8°C.
Shelf life Viferon® Shelf life - 2 years.
Terms of sale The drug is approved for use as an over-the-counter product.
Contacts for inquiries
FERON LLC (Russia)
123098 Moscow, Gamaleyi st. 18, bldg. And IEM im. N.F. Gamaleya Tel./fax,
Indications for the drug Viferon®
Ointment:
- viral (including herpetic) lesions of the skin and mucous membranes of various locations.
Gel:
— prevention and treatment of children with recurrent stenotic laryngotracheobronchitis and often suffering from acute respiratory diseases; - treatment of adults with chronic recurrent herpetic infections of various localizations.
Suppositories
In complex therapy: - various infectious and inflammatory diseases in children, incl. newborns and premature infants: ARVI, influenza, incl. complicated by bacterial infection, pneumonia (bacterial, viral, chlamydial), meningitis (bacterial, viral), sepsis, intrauterine infection (chlamydia, herpes, CMV infection, enteroviral infection, candidiasis, including visceral, mycoplasmosis); — chronic viral hepatitis B, C, D in children and adults, incl. in combination with the use of plasmapheresis and hemosorption, chronic viral hepatitis - a pronounced degree of activity and complicated by cirrhosis of the liver; - adults, incl. pregnant women with urogenital infections (chlamydia, CMV infection, ureaplasmosis, trichomoniasis, gardnerellosis, papillomavirus infection, bacterial vaginosis, recurrent vaginal candidiasis, mycoplasmosis), primary or recurrent herpetic infection of the skin and mucous membranes, localized form, mild and moderate course, incl. h. urogenital localization; - influenza and other acute respiratory viral infections and acute respiratory infections, incl. complicated by bacterial infection in adults.
What does Viferon help with?
The ointment is prescribed for use in cases of damage to the skin and mucous membranes, which is caused by the action of various viruses.
The gel is recommended to be taken for the prevention and treatment of acute respiratory infections in children.
Candles are prescribed for the following conditions and diseases:
- pathologies that are caused by inflammatory and infectious processes (can be used from the first days of babies’ lives);
- chronic viral hepatitis;
- pregnant women who have been diagnosed with urogenital infections;
- ARVI, flu and various colds.
Before use, be sure to consult a doctor.
Use during pregnancy and breastfeeding
Ointment:
since when applied externally and locally, the systemic absorption of interferon is low and the drug has an effect only in the affected area, it is possible to use the drug Viferon (ointment) during pregnancy and breastfeeding.
Gel:
Since when applied topically, the systemic absorption of interferon is low and the drug has an effect only in the lesion, it is possible to use the drug Viferon (gel) during pregnancy and breastfeeding. During lactation, do not use the drug on the breast and nipple area due to the ability of the drug to form a long-lasting film on the surface of the skin.
Suppositories:
the drug is approved for use from the 14th week of pregnancy. There are no restrictions for use during lactation.
Directions for use and doses
Ointment, externally and locally.
Apply a thin layer to the affected areas 3-4 times a day and rub in gently. Duration of treatment is 5-7 days. It is recommended to begin treatment when the first signs of damage to the skin and mucous membranes appear (itching, burning, redness). When treating recurrent herpes, it is advisable to begin treatment in the prodromal period or at the very beginning of the appearance of signs of relapse.
Gel, topically.
In order to prevent acute respiratory infections and recurrent stenosing laryngotracheobronchitis, children are applied with a hard swab to the surface of the tonsils 3 times a day for 3 weeks, 2 times a year; For therapeutic purposes, the drug is prescribed 5 times a day in the acute period of the disease (5-7 days), then 3 times a day for the next 3 weeks.
When treating adults with chronic recurrent herpetic infections of various localizations, treatment begins at the earliest possible date from the onset of relapse, preferably during the period of precursors. The drug is applied to the affected surface 3 to 7 times a day for 3-5 days. If necessary, the duration of the course is increased to 10 days. The number of repeat courses is not limited. The use of the gel is permissible during therapy with other drugs. When the gel is applied to the affected area, after 30-40 minutes a thin film is formed, onto which subsequent applications are made. If desired, the film can be peeled off or washed off with water.
When applying the gel to the affected mucous surface, it is first dried with a gauze swab.
Suppositories, rectally.
In the complex therapy of various infectious and inflammatory diseases in newborns, incl. premature: newborns, incl. premature babies with a gestational age of more than 34 weeks - Viferon 150,000 IU daily, 1 supp. 2 times a day after 12 hours. The course of treatment is 5 days. For premature newborns with a gestational age of less than 34 weeks - Viferon 150,000 IU daily, 1 supp. 3 times a day after 8 hours. The course of treatment is 5 days.
Recommended number of courses for various infectious and inflammatory diseases in children, incl. newborns and premature infants: influenza, ARVI, incl. complicated by bacterial infection - 1-2 courses; pneumonia (bacterial, viral, chlamydial) - 1-2 courses; sepsis - 2-3 courses, meningitis - 1-2 courses, herpetic infection - 2 courses, enterovirus infection - 1-2 courses, CMV infection - 2-3 courses, mycoplasmosis, candidiasis, incl. visceral, 2-3 courses. The break between courses is 5 days. According to clinical indications, therapy with Viferon rectal suppositories can be continued.
In the complex therapy of chronic viral hepatitis B, C, D in children and adults
For children with chronic viral hepatitis, the drug is prescribed in the following age-specific dosages: up to 6 months - 300,000-500,000 IU/day; from 6 to 12 months - 500,000 IU/day. At the age of 1 year to 7 years - 3,000,000 IU/m2/day; children over 7 years old - 5,000,000 IU/m2/day. The dose of the drug for each individual patient is calculated by multiplying the recommended dosage for a given age by the body surface area calculated using the nomogram for calculating body surface area by height and weight according to Garford, Terry and Rourke. Divide into 2 injections, round up to the dosage of the corresponding suppository. The drug is used 2 times a day after 12 hours for the first 10 days daily, then three times a week every other day for 6-12 months. The duration of treatment is determined by clinical effectiveness and laboratory parameters.
Children with chronic viral hepatitis of severe activity and cirrhosis of the liver before plasmapheresis and/or hemosorption are advised to use the drug for 14 days daily, 1 supp. 2 times a day every 12 hours (children under 7 years old - Viferon 150,000 IU; children over 7 years old - Viferon 500,000 IU).
Adults with chronic viral hepatitis - Viferon 3,000,000 IU, 1 supp. 2 times a day after 12 hours for 10 days daily, then three times a week every other day for 6-12 months. The duration of treatment is determined by clinical effectiveness and laboratory parameters.
In complex therapy in adults, incl. pregnant women with urogenital infections (chlamydia, CMV infection, ureaplasmosis, trichomoniasis, gardnerellosis, human papillomavirus infection, bacterial vaginosis, recurrent vaginal candidiasis, mycoplasmosis, primary or recurrent herpetic infection of the skin and mucous membranes, localized form, mild and moderate course, including urogenital localization)
For adults, with the above infections, except herpes, Viferon 500,000 IU, 1 supp. 2 times a day after 12 hours. Course - 5-10 days. According to clinical indications, therapy can be continued, the break between courses is 5 days.
For herpes infection - Viferon 1,000,000 IU, 1 supp. 2 times a day after 12 hours. The course of treatment is 10 days or more for recurrent infections. It is recommended to begin treatment immediately when the first signs of damage to the skin and mucous membranes (itching, redness, burning) appear. When treating recurrent herpes, it is advisable to begin treatment in the prodromal period or at the very beginning of signs of relapse.
In pregnant women with urogenital infection, including herpetic, in the second trimester of pregnancy (starting from 14 weeks) - Viferon 500,000 IU, 1 supp. 2 times a day every 12 hours for 10 days, then 1 supp. 2 times a day every 12 hours for 5 days. The preventive course is repeated every 4 weeks. If necessary, it is possible to conduct a treatment course before childbirth.
In the complex therapy of influenza and other acute respiratory viral infections and acute respiratory infections, incl. complicated by bacterial infection in adults: Viferon 500,000 IU, 1 supp. 2 times a day every 12 hours. The course of treatment is 5-10 days.
How to use Viferon suppositories for children
Candles are often given to newborns and babies who were born ahead of schedule. The drug in combination with specific treatment is taken depending on the existing infectious disease.
The course of therapy lasts 5 days. Use 1 suppository per day 2 times a day with the same time interval.
Premature babies whose gestational age is 34 weeks are given 1 suppository three times a day.
For acute respiratory viral infections and influenza, children may require 2 treatment courses. If the infection is associated with the herpes virus, then 2 courses of therapy will be required.
To put a suppository on a newborn, you need to place the baby on an oilcloth sideways, with his legs tucked under his tummy. It is better to use candles after bowel movements.