Endometriosis is a chronic multifactorial disease that affects every tenth woman of reproductive age. Establishing the exact prevalence of endometriosis is difficult due to the variety of symptoms and the invasiveness of reliable diagnostic methods. According to several sources [1, 2], the authors of which used different methodological approaches, endometriosis affects approximately 5.5 million women in the United States and Canada who are of reproductive age, as well as 16 million women in Europe.
The main symptoms of endometriosis are infertility and chronic pelvic pain. Chronic pain syndrome leads to decreased or temporary disability, which leads to serious social and economic consequences. In recent years, there has been an increase in interest in studying the impact of endometriosis on quality of life. The chronic nature of the disease creates problems in many aspects of the patient’s life - professional, social and personal spheres, emotionality and sexuality, leading to a significant decrease in the quality of life [3-6].
Regarding health care costs, there is an obvious need for surgery to make a definitive diagnosis and evaluate recurrences, as well as hospitalization for pain. The results of a multicenter study conducted by S. Simoens et al. [7] showed that the costs associated with endometriosis consist of the costs of surgical treatment (29%), diagnostic methods of examination (19%), hospitalization (18%) and medications (18%). Costs also depend on the extent of endometriosis, the presence of pelvic pain, infertility, and the duration of the disease before diagnosis. The cost of treating endometriosis in the United States (including pain management, hormonal therapy, specialist consultations, hospital and surgical treatment, days missed due to illness, and decreased reproductive potential) is about $20 billion per year, which significantly exceeds the cost of treating other chronic diseases. diseases such as Crohn's disease or migraine [8].
In addition, the majority of women with endometriosis had high levels of anxiety and depression [9, 10]. According to another study [11–14], the presence of endometriosis also correlates with anxiety and depression and often leads to dissatisfaction in the professional and socio-economic spheres, and a high level of psychological stress. It is known that from the moment of onset of chronic pelvic pain in women with endometriosis, social isolation begins to progress. All of the above emphasizes the need for a multidisciplinary approach to the treatment of this disease [15].
So, endometriosis has a great impact on the professional and personal lives of patients, and effective treatment of this disease can significantly improve the quality of life of women.
Currently, there is no unified strategy for selecting and prescribing hormonal therapy in patients with endometriosis in the world. Modern approaches to the combined treatment of endometriosis involve the use of gonadotropin-releasing hormone (GnRH) agonists as an effective drug treatment. However, the use of such therapy is accompanied by pronounced side effects caused by hypoestrogenemia, which reduces adherence to this type of treatment and makes its prolonged use impossible without additional prescription of add-back therapy.
The following postulate is important: for effective treatment of genital endometriosis, it is necessary that the hormonal drug suppresses ovulation, i.e., has an antigonadotropic property. However, without ovulation it is impossible to plan a pregnancy. At first glance, these are two mutually exclusive treatment strategies. However, according to approaches to the choice of therapy, it is first necessary to carry out hormonal therapy for endometriosis using drugs with antigonadotropic action, which will not only influence various aspects of the pathogenesis of the disease, but also maintain the desired pregnancy and prevent relapse of the disease. At the next stage of treatment, the patient is prescribed drugs against which pregnancy is planned (progestogens that do not have antigonadotropic properties).
According to the ESHRE (European Society for Human Reproduction and Embryology) guidelines, progestins “...can be considered as the therapy of choice for the treatment of endometriosis, as they are also effective in reducing the severity of the disease on the [laparoscopic] grading scale and in controlling pain, like danazol and GnRH agonists, which are cheaper and are characterized by a lower incidence of side effects” [16]. Therefore, given the good tolerability of progestogens, minimal metabolic effects and low cost, they can be considered as the drugs of choice [17].
It is known that the occurrence and progression of endometrioid heterotopias depend on the systemic and local level of estrogens and are accompanied by an increase in the local concentration of cytokines, chemokines and growth factors.
The mechanism of action of progestogens, which have antigonadotropic properties, is based on the effect on the hypothalamic-pituitary-ovarian axis, leading to anovulation, a decrease in the level of estrogen in the serum, decidualization and subsequent atrophy of endometrioid heterotopias, a decrease in the level of peritoneal markers of inflammation, as well as modulation of the immune response involved in pathogenesis of endometrioid disease [18—20].
Dienogest is a 4th generation progestogen and combines the pharmacological properties of the progesterone group and progesterone-like compounds, as well as 19-nortestosterone derivatives. The specificity of its action is complemented by minimal effects on metabolic parameters. Dienogest is the first gestagen that produces a clinically significant antiandrogenic effect without exhibiting estrogenic or androgenic activity [21]. Studies have shown that dienogest has an anovulatory and antiproliferative effect by suppressing the secretion of cytokines in the stroma of endometrial cells [17].
Thus, dienogest is characterized by good tolerability, a relatively moderate inhibitory effect on the secretion of gonadotropins, as well as a strong progestogenic effect on the endometrium and high bioavailability when taken orally (>90%) [22, 23].
Dienogest at a dose of 2 mg was specifically developed for the complex treatment of endometriosis and was studied in a special research program [24].
It is known that, in addition to estrogens, the progression of the disease is significantly influenced by inflammatory mediators, synthesized in large quantities in endometrioid heterotopias [25]. Progesterone receptors, which are the main point of application of dienogest, are involved in the regulation of the local inflammatory response, suppressing it through nuclear factor κB (NF-κB is a transcription factor responsible for the synthesis of pro-inflammatory cytokines) [26]. In addition, it has been determined that in endometrioid tissue there is an inverse relationship between NF-κB and the expression of progesterone B receptors [27].
Thus, the effectiveness of dienogest is largely due to its anti-inflammatory effect. In 2015, a group of scientists from Italy and Switzerland conducted a systematic review of the effect of dienogest on the inflammatory response in endometriosis lesions. Data from 15 studies were analyzed, on the basis of which reliable evidence was obtained of the anti-inflammatory effect of dienogest on epithelial cells in areas of endometriosis through progesterone receptors [28]. But when studying the stromal cells of endometrioid heterotopias, a similar effect of the drug was noted, despite blocking the progestogenic effect. In an experiment to create a model of endometriosis in rats [29], the antiproliferative effect of dienogest persisted even with the use of a progesterone receptor blocker, just as the increase in interleukin (IL)-8 messenger RNA expression associated with dienogest was not suppressed by the use of antigestagens [30]. The data obtained suggest the presence of additional mechanisms of the anti-inflammatory action of dienogest. This was confirmed in a 2015 study that compared changes in pelvic pain intensity and quality of life in patients taking dienogest and nonsteroidal anti-inflammatory drugs (NSAIDs). Patients with external genital endometriosis (EGE) treated with dienogest noted a 61% reduction in pain during menstruation on a visual analogue scale (VAS), as well as a significant improvement in quality of life. The use of NSAIDs did not have a significant effect on women's assessment of pain intensity and quality of life; 25% of patients in this group refused to participate during the study (compared to 5% in the dienogest group) [31].
In the pathogenesis of pain, one of the main roles is played by an increased level of inflammatory mediators in the endometriotic lesion, including prostaglandins, cytokines and growth factors. Recent evidence suggests that nerve growth factor (NGF) is one of the key mediators that mediates pain associated with endometriosis. NGF promotes an increase in sensory neurons in the focus of endometriosis and an increase in the number of nociceptive receptors on them. The activity of this factor is regulated by inflammatory cytokines such as tumor necrosis factor α (TNF-α) and IL-1β [22, 23].
In 2014, Japanese scientists [32] in an in vivo
It has been convincingly demonstrated that dienogest reduces the levels of TNF-α and IL-1β and thereby suppresses the expression of NGF in endometriotic tissue, helping to reduce pain. Immunohistochemical analysis of endometrioid heterotopias of women receiving dienogest revealed its effect on the level of enzymes involved in the local metabolism of estrogens in pathological lesions. Dienogest suppresses excessive estrogen production by inhibiting aromatase and 17β-hydroxysteroid dehydrogenase [33]. One recent study [34] determined that dienogest suppresses the level of midkain growth factor in the peritoneal fluid of patients with endometriosis. In addition, dienogest also has a suppressive effect on midkine expression in cultured endometrial stromal cells in patients with endometriosis. It is important to note that medroxyprogesterone acetate and norethisterone acetate did not demonstrate a similar effect. The data obtained may serve as another explanation for the effectiveness of dienogest therapy.
Thus, dienogest is currently a pathogenetically substantiated drug of choice for the treatment of genital endometriosis. However, experience with the routine use of this type of therapy in clinical practice and long-term follow-up of patients are important.
The purpose of the study was to evaluate the effectiveness and tolerability of long-term use of dienogest 2 mg, as well as the possibility of implementing reproductive plans and preventing relapses in patients with NGE.
Results and discussion
The results of observation of 937 patients with NGE who received a 6-month course of therapy with dienogest 2 mg were analyzed; 572 patients subsequently continued this type of treatment. In all patients, the diagnosis of NGE was established on the basis of laparoscopy and confirmed by the results of histological examination. According to the classification of the American Fertility Society (R-AFS), degree I of the disease was diagnosed in 66 (7%) patients, degree II - in 223 (23.8%), degree III - in 254 (27.1%) and degree IV — in 394 (42.1%). Thus, the majority - 648 (69.2%) patients - had widespread endometriosis. Retrocervical endometrioid infiltrate was observed in 246 (26.3%) patients, extragenital endometriosis (appendix, colon, bladder, diaphragm) was detected in 81 (8.7%) patients. In 628 (67%) women the disease was recurrent. One of the problems in treating endometriosis is the decrease in ovarian reserve after surgical treatment, which in turn can have a negative impact on reproductive function. The most commonly identified predictor of low ovarian response to ovulation stimulation can be considered a decrease in the level of anti-Mullerian hormone (AMH) in the peripheral blood. It has been proven that endometrioid cysts reduce ovarian reserve, and cystectomy has an even greater negative effect on it, which is reflected in a decrease in AMH levels. Thus, the potential impact of many common surgical procedures on ovarian reserve is significant. In patients with a recurrent course of endometriosis, 402 (64%) patients had a history of GnRH therapy. Based on determination of the level of AMH in the blood serum, a reduced ovarian reserve was noted in 694 (74.1%) women with NGE; the average AMH level was 0.56±0.29 ng/ml.
According to the literature [35], endometriosis is found in 15–80% of women who have undergone laparoscopic surgery for chronic pelvic pain. The structure of complaints of patients with endometriosis is dominated by pain, which can be expressed in the form of dysmenorrhea, dyspareunia, chronic pelvic pain, dysuria and dyschizia. Studies have shown that in half of the cases, women undergoing surgery for endometriosis subsequently continue to suffer from dyspareunia, which leads to problems in their sexual life and relationships with their partner [36]. Based on a study of the subjective assessment of health status (SF-36v2) in 8 key aspects and the overall result, a significant decrease in general health indicators was found in patients with endometriosis compared to the control group [37]. It is important to note that the severity of the negative impact of endometriosis was comparable to that in patients with Crohn's disease [37].
A multicenter study [38] of the occupational activities of people with endometriosis found that symptoms of the disease have a negative impact on work productivity, which corresponds to the loss of about one working day per week. Another study [14] found that 85% of patients with endometriosis reported a clear decline in the quality of their work, 19% reported being unable to work due to pain, and 69% of patients reported continuing to work despite pain. It is known that recurrence of dysmenorrhea within a year after laparoscopic intervention was observed in almost every third patient who did not receive drug treatment [39].
Therefore, the use of hormone-modulating therapy to eliminate pain is one of the most important tasks. Before starting therapy with dienogest 2 mg, dysmenorrhea was observed in 772 (82.4%) patients with NGE, dyspareunia in 439 (46.9%), complaints of pelvic pain not associated with the menstrual cycle were reported in 384 (41%), dysuria in the time of menstrual reaction was typical for 28 (3%), dyschisia - for 37 (3.9%) patients.
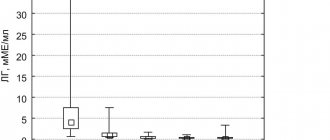
During a 6-month course of using dienogest, a significant decrease in pain was noted: the severity of dysmenorrhea decreased by 4.4 times (18.7%), dyspareunia by 2 times (23.5%), and pelvic pain by 2.5 times ( 16.4%), dysuria - 3 times (1% during treatment), dyschisia - 3.4 times (1.2%). We also assessed the dynamics of pain in 572 patients with NGE during prolonged use of dienogest 2 mg. The duration of therapy ranged from 10 to 37 months (average 29.5±8.2 months). There was a further significant decrease in the severity of pain: dysmenorrhea by 89%, pelvic pain by 85.8%, dyspareunia by 81.6%, dyschizia by 98.9%, dysuria by 99.3% (see figure).
Dynamics of pain during dienogest therapy.
Known and expected side effects during progestogen therapy are menstrual irregularities, manifested by irregular bleeding of varying duration and abundance. To increase adherence to therapy, before starting the use of dienogest 2 mg, a conversation was held with patients regarding possible changes in the nature of bleeding. When dienogest 2 mg was prescribed for 6 months, the regular menstrual cycle was preserved in 72 (12.6%) patients, but menstruation became significantly more scanty. Menstrual irregularities such as “intermenstrual” spotting were observed in 261 (45.6%) women, episodes of “breakthrough bleeding” were observed in 23 (4.0%) women. A fairly high frequency of amenorrhea was noted in this group - in 215 (37.6%) patients. Against the background of prolonged administration of dienogest 2 mg, a regular menstrual cycle was observed in only 6.8% of patients. With increasing duration of therapy, a decrease in the intensity, frequency and duration of intermenstrual bleeding was observed - in 48.1% of patients, menstrual irregularities such as opsomenorrhea were noted in 5.4%, and the number of patients with amenorrhea increased - 39.7%. Episodes of breakthrough bleeding during prolonged use of dienogest were observed in 0.5% of patients with NGE.
Interesting are the data on the implementation of reproductive function in 314 patients with NGE with infertility aged from 27 to 39 years after therapy with dienogest 2 mg. Primary infertility was noted in 216 (68.8%) patients, secondary infertility - in 98 (31.2%). The duration of primary infertility was 6.3±2.8 years, secondary - 5.4±2.3 years. A history of recurrent disease before the prescription of dienogest 2 mg was noted in 135 (43%) patients, reduced ovarian reserve - in 186 (59.2%). Based on our own experience, we recommend planning a pregnancy in a natural cycle or entering into an in vitro fertilization (IVF) protocol immediately after finishing progestogen therapy. After hormone-modulating therapy with dienogest, pregnancy occurred in 106 (33.8%) women; of these, in the natural cycle in 41.5% and as a result of the IVF procedure in 58.5% of patients. In 33% of women, pregnancy occurred 3-5 cycles after discontinuation of dienogest 2 mg. Due to the fact that dienogest has a moderate effect on the level of gonadotropins, its use is justified in patients with reduced ovarian reserve.
Clinical example
Patient B
., 32 years old. In 2008, a diagnosis of stage IV OGE was made. Bilateral endometrioid cysts. Retrocervical endometrioid infiltrate. Laparoscopy was performed. Bilateral resection of the ovaries for endometrioid cysts, excision of the retrocervical endometrioid infiltrate. For 2 years she took a combined contraceptive pill (COC) containing 30 mcg ethinyl estradiol.
In the period from 2010 to 2013, three IVF attempts were made without effect.
In 2014, a repeat laparoscopy was performed. Diagnosis of stage III OGE, relapse. Endometrioid cyst of the left ovary. Retrocervical endometrioid infiltrate (relapse). Resection of the left ovary, excision of the retrocervical endometrioid infiltrate and foci of endometriosis were performed.
After the operation, she received hormonal therapy: GnRH at a dose of 3.75 mg intramuscularly after 28 days No. 6. After completion of therapy, the patient entered into an IVF protocol - without effect.
In 2015, due to the appearance of pain and relapse of endometriosis, a third laparoscopy, adhesiolysis, and right-sided cystectomy were performed. Diagnosis of stage IV OGE, relapse. Right ovarian cyst. An anti-adhesion barrier has been installed.
The AMH level after surgery (4th day of the menstrual cycle) was 0.42 ng/ml, FSH - 13.7 mIU/ml.
The patient was prescribed dienogest at a dose of 2 mg once a day for 6 months. Immediately after the end of therapy, the patient underwent stimulation of superovulation in the ART program. Pregnancy occurred, which was accompanied by phenomena of threatened miscarriage in the first and second trimesters. The patient was delivered by cesarean section at 38–39 weeks.
The following clinical case deserves attention, which rarely ends in pregnancy in routine practice.
Patient B
., 36 years old, complained of primary infertility for 7 years, dark brown spotting before and after menstruation, dyspareunia. Menarche from 11 years of age, regular menstrual cycle, every 27-30 days, 5-6 days at a time, painful menstruation.
In 2009, laparoscopy was performed. Left-sided tubectomy. Diagnosis: chronic bilateral salpingoophoritis. Pyosalpinx on the left. Results of histological examination: phlegmonous salpingitis. I received COCs for a year. Hysterosalpingography was performed and the patency of the right fallopian tube was preserved.
From 2011 to 2013, 6 cycles of ovulation stimulation with gonadotropins were performed - without effect. Results of hormonal studies: FSH - 8.4 mIU/ml, AMH - 0.64 ng/ml.
In 2013, hysteroresectoscopy was performed due to a glandular endometrial polyp. Subsequently, 4 cycles of ovulation stimulation were carried out - without effect.
In 2015, she contacted the Federal State Budgetary Institution “Research Institute of AGiR named after. BEFORE. Otta." Taking into account the patient's complaints and medical history, laparoscopy, hysteroscopy and immunohistochemical examination of the endometrium were performed.
Diagnosis: stage II OGE. Primary infertility 7 years. Chromohydrotubation on the right - the fallopian tube is patent. Foci of endometriosis were excised.
Based on the results of an immunohistochemical study of the endometrium, chronic endometritis.
Results of hormonal studies (on the 3rd day of the menstrual cycle): FSH - 10.1 mIU/ml, AMH - 0.32 ng/ml. Given the reduced ovarian reserve, the patient was recommended to take dienogest 2 mg for 6 months. The patient categorically refused the IVF procedure and insisted on planning a pregnancy in a natural cycle. The husband's spermogram results show normozoospermia.
Two cycles after completing the course of dienogest therapy, the patient became pregnant on her own, currently at 22-23 weeks.
Of course, every drug can cause side effects. During treatment with dienogest 2 mg, mood changes were most often observed - in 143 (15.3%) women, mastodynia - in 132 (14%), weight gain - in 91 (9.7%), and cne vulgaris
- in 78 (8.3%), complaints of headache or dizziness - in 34 (3.6%). The above side effects were mild or moderate and were not a reason for early termination of treatment.
The VISADO multicenter study [40] on the use of dienogest 2 mg in adolescents with pain associated with endometriosis reported safety and good tolerability of the drug in this subpopulation. The severity of pain associated with endometriosis decreased from 64.3 to 9 points (based on VAS). In addition, this study examined bone mineral density (BMD) during the use of dienogest. After a year of treatment, there was a minimal decrease in BMD (by 1.2%), gradually recovering over the next 6 months. One patient experienced a 6.6% decrease in bone mass, which was assessed as an unexpected adverse reaction. This study [40] showed the safety and good tolerability of dienogest in adolescents with symptoms of endometriosis.
It is known that modern women have individual risk factors for decreased BMD associated with lifestyle and nutrition, as well as with hereditary predisposition and drug therapy. A number of drugs for the treatment of endometriosis (GnRH agonists, anti-Liberin hormones, aromatase inhibitors and depot forms of medroxyprogesterone acetate) have an effect on reducing BMD. It should be recalled that in the group of patients presented in our study, a history of therapy with GnRH agonists was noted in 64% of patients. Therefore, with prolonged use of dienogest 2 mg, long-term administration of calcium supplements with vitamin D3 is advisable, which is what we use in routine practice. The results of repeated osteodensitometry (lumbar spine and proximal femur) confirmed the absence of significant changes in BMD during such therapy. In patients with diagnosed osteoporosis or severe osteopenia, established before the start of the use of dienogest 2 mg, bisphosphonates (mainly ibandronic acid) are prescribed along with the mandatory intake of calcium supplements with vitamin D3 and osteotropic minerals.
Undoubtedly, special attention should be paid to modern aspects of prescribing COCs for the treatment of endometriosis. It is known that the mechanism of action of COCs is carried out through inhibition of the hypothalamic-pituitary-ovarian system, as a result, folliculogenesis and ovulation are suppressed in the ovaries, and estradiol production is reduced. The use of COCs causes regression of proliferative processes in the first phase of the menstrual cycle and incomplete secretory transformation in the second. With long-term use of estrogen-progestogen contraceptives, involution of the glandular epithelium of the endometrium occurs, and often its atrophy, the stroma undergoes decidual transformation (a state of “pseudo-pregnancy”), the severity of which depends on the gestagenic component.
In recent years, data have appeared in the literature about the possible negative impact of COCs on the course of endometriosis. Considering that endometriosis is characterized by “progesterone resistance”, and the main active component in COCs is progestogen, a number of researchers warn about the possible incomplete interaction of the hormone-receptor complex and, as a consequence, the insufficient progestogenic effect of this component and the negative estrogenic stimulation associated with the use of estrogen component in the contraceptive drug. Despite this, quite often, in the outpatient stage of treatment, patients after surgery are prescribed COCs. Long-term use of COCs prescribed in a standard cyclic regimen is accompanied by so-called “masks of false well-being” of endometriosis, manifested by a temporary decrease in symptoms, primarily pain (dysmenorrhea, dyspareunia, pelvic pain).
According to data obtained from the Federal State Budgetary Institution “Research Institute of AGiR named after. BEFORE. Otta”, the use of a dienogest-containing COC with 30 mcg ethinyl estradiol in a cyclic mode after surgical treatment of NGE is characterized by a high frequency and severity of disease relapses [41]. It is important to note that relapses of NGE, established on the basis of repeat laparoscopy after cyclic use of the above-described COC, in most cases were accompanied by excision of endometrioid infiltrates. During the first laparoscopy, no endometrioid infiltrates were detected [41]. One meta-analysis also showed that 95% of patients receiving COCs for severe primary dysmenorrhea were subsequently diagnosed with deep infiltrative endometriosis [42].
Obviously, COCs are not the cause of endometriosis - they only “mask” its manifestations, reducing the severity of pain. As already noted, all patients before the prescription of this group of drugs had an important pathognomonic symptom of endometriosis - severe dysmenorrhea. The data obtained show the inappropriateness of using COCs in a cyclic mode in patients with NGE. In addition, it is known that when using COCs in a standard cyclic mode with a 7-day break, already from the 4th day of this break the follicle grows, which, when reaching a size of 8-10 mm, has FSH-dependent aromatase activity and, therefore, estrogenic activity. activity. Thus, according to research [43], it is currently impossible to exclude the possibility that the protective effect of COCs (temporary reduction of pain symptoms) in endometriosis leads to untimely, delayed surgical intervention. COCs are known to reduce the production of endogenous estradiol due to their antigonadotropic effect, but the question is whether the estrogenic component will stimulate disease progression?
The above is also confirmed by the following clinical example:
Patient B
., 34 years old, applied to the Federal State Budgetary Institution of Agir.
BEFORE. Otta, 03/31/16 with complaints of dysmenorrhea, dysuric phenomena during menstruation, spotting before and after menstruation, severe dyspareunia. From the anamnesis it is known: menarche since the age of 13, the menstrual cycle is regular, every 26-28 days, every 5 days, menstruation is painful. Sexual life since 24 years old. There were no pregnancies. Due to algodismenorrhea, from the moment of menarche she received COCs (for 20 years!)
.
In 2009, after taking COCs for 14 years, laparoscopy, bilateral cystectomy, and removal of a single myomatous node were performed. Postoperative diagnosis: stage IV OGE. Uterine fibroids. Adenomyosis. Histological conclusion: endometrioid cysts, uterine leiomyoma. In the postoperative period, she received the COC Zhanine for 6 months, then Femoden as a contraceptive for 3 years.
In 2012, repeated laparoscopy, bilateral cystectomy, adhesiolysis, salpingolysis, and chromohydrotubation were performed. Diagnosis: IV degree NGE, relapse. Bilateral ovarian cysts. Endometriosis of the pelvic peritoneum. Adenomyosis. Adhesive process of 3-4th degree. With chromohidrotubation, the right fallopian tube is obstructed, the left fallopian tube is patent. Histological conclusion: endometrioid ovarian cysts. The COC Evra was prescribed transdermally, which the patient received until November 2015. After discontinuation of the COC in connection with pregnancy planning, complaints began about the absence of a menstrual cycle for 6 months, hot flashes, and sweating during the day. She noted the resumption of the menstrual reaction on 07.16 after 3 months against the background of herbal medicine.
Objectively
: height 173 cm, body weight - 73 kg, body mass index - 24.5 kg/m2. Blood pressure 120/80 mm Hg. Breasts with moderately pronounced signs of diffuse fibroadenomatosis. There is no lactororrhea.
Gynecological examination
.
In the speculum: cervix with slight ectopia. The discharge is mucous-whitish and scanty. PV
: uterus in interposition, small size, heterogeneous consistency, mobile, painless. Palpation of the posterior fornix is painful. To the left posterior to the uterus, appendages are palpated in the form of a tight-elastic formation, 6 cm in diameter, in adhesions. On the right, the appendages cannot be clearly palpated. Signs of a rough cicatricial adhesive process in the pelvis.
Hormonal study data from 07.16 (3rd day of the menstrual cycle): AMH - 0.22 ng/ml, FSH - 89.80 mIU/ml, LH - 46.92 mIU/ml, prolactin - 263 µIU/ml, progesterone - 1.10 ng/ml, estradiol - 214 pg/ml. CA 125 from 03.16 - 69.40 IU/ml.
According to magnetic resonance imaging (MRI) of the pelvic organs dated 05.16: condition after repeated surgical interventions. MRI signs of diffuse adenomyosis. MRI signs of a left ovarian cyst, 52x35 mm in size, with hemorrhagic contents (probably endometrioid). The area along the vesicouterine fold on the left is most likely represented by a focus of endometriosis. Signs of adhesions in the pelvis.
Clinical diagnosis: OGE, relapse? Left ovarian cyst (endometrioid). Adenomyosis. Bladder endometriosis? Hypergonadotropic normoprolactinemic ovarian failure. Aggravated gynecological history (twice - history of bilateral ovarian resection, grade IV OGE, recurrent, myomectomy).
The given clinical example once again emphasizes the need to determine the ovarian reserve in patients after surgery on the ovaries in order to select hormone-modulating therapy and implement reproductive function.
Undoubtedly, it is impossible to refuse the use of COCs in patients with endometriosis, since many patients need effective contraception, regulation of the menstrual cycle, and correction of pain. However, the question of the need for a prolonged regimen of COC use and the minimum dose of the estrogen component in their composition in patients with NGE is of fundamental importance [44].
Therefore, for the treatment of endometriosis, only monophasic drugs are used, with a minimum dose of the estrogen component; it is possible to choose drugs with an increased dose of the progestin component and it is recommended to use the so-called “prolonged” regimen of COCs, although this regimen is not registered with all COCs. It is possible to use COCs containing estrogens that are bioidentical to natural ones, for example, a drug containing dienogest with 17β-estradiol.
It should be remembered that the effectiveness of drug therapy and the frequency of relapses also depend on the quality of the surgical treatment performed. Based on data from the Federal State Budgetary Institution “Research Institute of AGiR named after. BEFORE. Otta" conducted a retrospective analysis of the effectiveness of various types of hormonal therapy in patients with NGE after surgical treatment. 573 patients received GnRH therapy for 6 cycles, 464 - dienogest 2 mg for 6 months, 176 - COCs in the standard regimen (mainly containing 30 mcg ethinyl estradiol), 6-8 months, 158 - aromatase inhibitors against the background of progestogen (linestrenol), 6 months . The frequency of disease relapses in the group of patients receiving COCs for the treatment of endometriosis was the highest - 67.1%; after GnRHa therapy - 14.3%; after using dienogest 2 mg - 11.9%; after therapy with aromatase inhibitors against the background of progestogens - 8.2%.
Modern progestogens in the treatment of endometriosis (review of international studies)
Doctor of Medical Sciences, Prof. G.E. CHERNUKHA Problems of Reproduction 2012;No. 4:68-71 Scientific Center of Obstetrics, Gynecology and Perinatology named after. IN AND. Kulakova Ministry of Health and Social Development (director - Academician of the Russian Academy of Medical Sciences G.T. Sukhikh)
Endometriosis can be considered a common gynecological disease, occurring in 6-10% of women of reproductive age. In chronic pelvic pain and infertility, it is observed in 40-70% and 30-50%, respectively (1,2). Endometriosis is a benign proliferation of tissue, functionally and morphologically similar to normal endometrium, which is characterized by increased local production of estrogens and sensitivity to them, as well as resistance to progesterone. Concomitant inflammatory process, immunological dysregulation, inhibition of apoptosis, activation of angiogenesis are pathogenetic factors that promote the survival and growth of endometrioid implants (1).
Surgery is considered first-line treatment, but the risk of recurrence is high (40-50% within 5 years) (3). Hormone therapy is considered an alternative to surgical treatment for symptoms characteristic of endometriosis and the absence of absolute indications for surgical treatment; hormone therapy is also used as postoperative adjuvant therapy, which reduces the risk of relapses. Hormone therapy for endometriosis is based on two main strategies. This is either the creation of a “pseudomenopause” state due to blockade of the pituitary-ovarian system with the initiation of hypoestrogenism, leading to atrophy of endometriosis foci (GnRH agonists and antagonists, aromatase inhibitors), or the induction of a pseudodecidualization state with subsequent blockade of proliferation and atrophy of endometriosis foci (COCs, progestogens , selective progesterone receptor modulators). Until recently, GnRH agonists were considered the “gold standard” of drug therapy. However, when they are used for more than 6 months (without “return therapy” with estrogen), undesirable hypoestrogenic effects occur, such as hot flashes, vaginal dryness, headache, decreased libido, loss of bone mineral density (BMD) (4). In recent years, attention to GnRH antagonists and aromatase inhibitors has increased, but their effectiveness and safety require in-depth study (5,6). Endometriosis is traditionally considered a persistent, relapsing disease that requires long-term treatment, so you need to think about the safety and degree of tolerability of drugs; in this regard, leading experts of International Societies include COCs and progestogens as first-line therapy (7,8). But only a few progestogens are currently approved by regulatory authorities for the treatment of endometriosis; these drugs in our country include dienogest at a dose of 2 mg per day. Benefits of progestogens.
Progestogens are used to treat and prevent relapses of endometriosis, since they are as effective as GnRH agonists and COCs. Firstly, progestogens have a “central effect”, inhibiting the hypothalamic-pituitary-ovarian system, as a result of which the secretion of estrogen is reduced, and secondly, with continuous use they have a direct effect on the foci of endometriosis, causing differentiation of stromal cells (decidualization) and atrophy of the glandular endometrial component. Third, progestogens activate the enzyme 17 beta-hydroxysteroid dehydrogenase, which converts estradiol into “weak” estrone, this changes the estrogen balance at the local level (9). Progestogens also have a blocking effect on the key components of pathogenesis - proliferation, angiogenesis and inflammation. Dienogest is a fourth generation progestogen.
Dienogest (DNG) is classified as a fourth-generation progestagen; it combines the properties of 19-nortestosterone derivatives (proven high selectivity and antiproliferative activity, short half-life from the bloodstream, high bioavailability when administered orally, low risk of accumulation when taken daily) and the properties of progesterone (good tolerability , the absence of negative metabolic, vascular and hepatic effects (10-13). The peculiarity of the chemical structure of DNG - the absence of an ethynyl radical, probably determines its metabolic neutrality. This is an advantage that allows long-term use of DNG. Currently, DNG is, in fact, the only oral progestogen, the effectiveness and safety of which has been proven in two parallel clinical programs performed in Japan and Europe (compared with placebo or GnRH agonists).Clinical studies of dienogest.
Randomized controlled clinical trials lasting 12 to 24 weeks have provided information on the optimal effective dose of the drug (2 mg/day) and confirmed its good tolerability. According to the visual analogue scale (VAS), this dose relieves pain caused by endometriosis: dysmenorrhea, dyspareunia, diffuse pelvic pain; this effect is associated with atrophy of endometrioid lesions on the rAFS scale (14,15).
Noteworthy are the results of three independent studies, lasting from 16 to 24 weeks, that directly compared DNG with various forms of GnRH agonists, the standard therapy for endometriosis (16–18). In a European study (17), 252 patients with endometriosis received DNG (2 mg/mg) or depot leuprolide acetate (LPA) at a standard dose (3.75 mg intramuscularly every 4 weeks). Treatment was accompanied by a reduction in pelvic pain of similar duration; by week 24, the decrease in the average VAS score was 47.5 mm for the DPG and 46.0 mm for the LPA. There was also a marked improvement in physical and mental well-being according to questionnaire data (modified health questionnaire - SF-36). Another multicenter study compared the effectiveness of DNG (2 mg/day) and triptorelin depot (3.75 mg intramuscularly, monthly) as postoperative treatment in 142 women with stage II-IY endometriosis over a 16-week period (16). Both drugs were equally effective in relieving symptoms and signs of endometriosis, including the prevalence of endometrioid lesions during repeat laparoscopy (there was no significant difference in the severity of endometriosis and the severity of adhesions). These results suggest that DNG is as effective as triptorelin, even against fairly severe forms of endometriosis, the hypothesis of the superiority of one drug over the other turned out to be untenable. The third, 24-week, double-blind, multicenter study in Japan compared the efficacy and safety of DNG (2 mg/day) and intranasal buserelin (900 μg/day) in 271 patients with endometriosis (18). DNG was also effective as buserelin; a decrease in the severity of pain and the prevalence of endometriosis foci was detected, confirmed by repeat laparoscopy. The greatest improvements in quality of life indicators in both groups concerned a decrease in physical pain with a tendency towards more pronounced relief of this symptom against the background of NH.
In general, in these direct comparative studies, the number, nature and severity of side effects during treatment with DNG and GnRHa agonists were comparable, with the exception of hypoestrogenic manifestations, which were significantly more common when taking GnRHa agonists. In the group of women receiving LPA, a decrease in the average BMD in the lumbosacral spine was detected, while against the background of NH there was a slight increase; after 6 months, significant intergroup differences were established (17). In the study population of Japanese women, a decrease in BMD was noted in both groups, but statistically significant differences were found only with buserelin (18). When using LPA, the number of days with “hot flashes” averaged 4.7 per week – with DNG it was only 0.82 (17). In another study, when taking triptorelin, “hot flashes” were observed in 61.2% of women, while with dienogest – only in 9.6% (16). According to the hypothesis proposed by Barbieri (19) back in 1992, there is a certain fairly low threshold concentration of estrogen in the blood serum (30-60 pc/ml), which does not allow stimulating the growth of endometriosis foci and prevents the appearance of signs of hypoestrogenism, ensuring stable BMD. Preclinical and clinical studies have shown that DNG suppresses ovulation with a moderate decrease in ovarian estradiol synthesis, levels of the latter being within the “therapeutic” window of estrogen action (10,11). This does not lead to increased proliferation in endometriotic lesions, but avoids the development of the above-mentioned symptoms of estrogen deficiency.
Dienogest is a drug for long-term treatment of endometriosis
Women who completed the European placebo-controlled 12-week study (17) were asked to participate in an open-label extension 53-week clinical trial, and the majority (89%) consented (20). The total duration of DNG treatment was 65 weeks, and in addition to improvement in the women's condition, a significant further reduction in pain intensity was observed during the placebo-controlled phase of the trial. The results of the European long-term study are consistent with data obtained from 52 weeks of DNG (2 mg/day) in 135 women with confirmed endometriosis in Japan (21). General improvement in well-being was determined by the severity of five subjective symptoms (pain in the lower abdomen, lower back pain, dyschezia, dyspareunia and pain on vaginal examination) and two objective signs (involvement of the peritoneum of the Douglas pouch and uterine mobility in the pathological process). It is important to note that moderate or significant improvement in general condition was detected in 72.5% of patients after 24 weeks of therapy and in 90.6% after 52 weeks of treatment with DNG. This means that the effectiveness of treatment was not only stable, but also increasing, and a state of resistance did not develop. The therapeutic effect persisted for at least 6 months after discontinuation of DNG.
Thus, the results of clinical programs consisting of dose selection of the drug, comparative studies with GnRHa and long-term studies (52-65 weeks) demonstrated the high effectiveness of DNG (2 mg/day) for the treatment of endometriosis, significantly superior to placebo and comparable to GnRHa. It should be especially emphasized that in all studies, side effects due to ONH were minor or moderate. The incidence and intensity of uterine breakthrough bleeding decreased with duration of treatment and was not reflected in the extremely low rates (4.4% and 5%) of discontinuation of further treatment (17,18).
Dienogest and fertility issues
Endometriosis is known to have a negative impact on fertility, with an incidence of 30-50% among infertile women. Management of infertility caused by endometriosis causes significant difficulties, since until now, despite a huge number of scientific studies, there is no clear understanding of the pathophysiological mechanisms that prevent pregnancy, especially in small and moderate forms of the disease. For a long time, there was no effective treatment for endometriosis that was well tolerated and had a predictable effect on ovulation and the timing of restoration of fertility after cessation of treatment. The results of a meta-analysis of many RCTs (22) showed that ovarian suppression to improve fertility in mild to moderate forms of endometriosis is ineffective and is not recommended for patients with the sole purpose of restoring fertility. In such cases, treatment should include surgical removal of endometriotic lesions and adhesions to restore normal anatomy of the pelvic organs or the use of assisted reproductive technologies when pregnancy does not occur on its own.
International recommendations of recent years with a high level of evidence provide evidence that laparoscopy increases the likelihood of pregnancy, while subsequent hormone therapy for minor forms of endometriosis does not significantly increase fertility and may lead to a delay in pregnancy. Interesting data is presented in the article by M. Cosson et al. (16) in 86 women with endometriosis and infertility from among all participants. As postoperative treatment for 16 weeks, they were prescribed DNG (2 mg/day) (n=45) or triptorelin according to the standard regimen (n=41). After discontinuation of DNG, pregnancy occurred spontaneously in 33% of cases, after discontinuation of triptorelin - in 29%, and resulted in childbirth in 29% and 22%, respectively. This indicates that in the presence of infertility in women with common forms of endometriosis, DNG therapy is not inferior in effectiveness to treatment with GnRHa and even slightly reduces the risk of pregnancy loss. There is evidence in the literature that in women with endometriosis, especially with relapses of the disease, suppression of ovarian function with GnRH agonists for 3-6 months increases the success rate of IVF (23). Taking into account the similar effectiveness of DNG and some additional positive effects, it seems possible to prescribe this drug before IVF programs, the results of comparative clinical studies will prove the feasibility of this approach.
It should also be noted that many women with endometriosis have an ovulatory menstrual cycle and require contraception. There is now evidence that DNG (2 mg/day) provides a robust antiovulatory effect through apoptosis of granulosa cells of the dominant follicle (11,13,24). Inhibition of ovulation is also carried out due to a weak central effect (suppression of FSH and LH) and a moderate decrease in the level of estrogen in the blood serum, which is an advantage of DNG compared with various forms of GnRH agonists (10,11). Despite the suppression of ovulation, DNG has not yet been registered as a contraceptive, so women must be advised to use non-hormonal methods of birth control during the treatment period. Many studies have obtained convincing data on the rapid restoration of fertility (on average 30 days) and on successful pregnancy in women who received DNG (2 mg/day) for a sufficiently long time (for up to one year) for the treatment of endometriosis (20,21,25) .
Conclusion
Resistance to progesterone is considered one of the key pathogenetic mechanisms for the development of endometriosis, since there is no progestogenic effect antagonistic to estrogen. This argues in favor of choosing a progestogen that has not only pronounced antiproliferative activity, but also an effect on other pathogenetic components of the disease and metabolic neutrality, allowing for long-term therapy. Numerous experimental and clinical studies have revealed many of the mechanisms of action of DNG and assessed its effectiveness for the treatment of endometriosis, which exceeds that of other progestogens, as well as its safety. It has been proven that this progestogen, similar in effectiveness to GnRH agonists, can be used long-term for the treatment of pain and the prevention of relapses of endometriosis without causing adverse hypoestrogenic side effects. DNG inhibits ovulation for the period of treatment, but does not have a negative effect on the restoration of fertility after discontinuation of treatment. There is an obvious relationship between endometriosis and infertility, and a significant decrease in the fertility index has been proven. There are promising studies on the effectiveness of using DNG in women with endometriosis and infertility before IVF.
The high effectiveness of DNG therapy, few side effects, and the possibility of long-term use contribute to an informed choice of optimal treatment for endometriosis. It is significant that all the scientific studies presented above provide information about high adherence to therapy and the desire of most women to continue treatment. Overall, the results of research programs in Europe and Japan indicate that DNG, among other hormone therapy methods, is a promising long-term treatment for endometriosis.
Links
1. Giudice LC. Endometriosis. N Engl J Med 2010;362:2389-98.
2. Littman E, Giudice L, Lathi R, Berker B, Milki A, Nezhat C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fertil Steril 2005;84:1574-8
3. Guo SW. Recurrence of endometriosis and its control. Hum Reprod Updare 2009;15:441-61
4. Sagsveen M, Farmer JE, Prentice A, Breeze A. Gonadotrophin-releasing hormone analogues for endometriosis: bone mineral density. 2003;(4):CD001297
5. Küpker W, Felberbaum RE, Krapp M, Schill T, Malik E, Diedrich K. Use of GnRH antagonists in the treatment of endometriosis. Reprod Biomed Online 2002;5:12–16
6. Racine AC, Legrand E, Lefebvre-Lacoeuille C et al Treatment of endometriosis by aromatase inhibitors: efficacy and side effects. Gynecol Obstet Fertil 2010;38:318-23
7. Leyland N, Casper R, Laberge Ph, Singh SS et al. Endometriosis: diagnosis and management. SOGC Clinical and practical guideline. Journal of Obstetrics and Gynecology Canada 2010;32(7):1-28
8. Management of endometriosis. Practical Bulletin No.114. The American College of Obstetricians and Gynecologists. Obstet Gynecol 2010;116:223-36
9. Bulun SE Endometriosis. N Engl J Med 2009;360:268–279
10. Sasagawa S, Shimizu Y, Kami H, Takeuchi T, Mita S, Imada K, et al. Dienogest is a selective progesterone receptor agonist in transactivation analysis with potent oral endometrial activity due to its efficient pharmacokinetic profile. Steroids 2008;73:222-31.
11. Klipping C, Duijkers I, Faustmann TA, et al. Pharmacodynamic study of four oral dosages of dienogest. Fertil Steril 2010: 94 (suppl): S181
12. Sitruk-Ware R. New progestagens for contraceptive use. Human Reproduction Update, Vol.12, No.2 pp. 169–178, 2006
13. Oettel M, Breitbarth H, Elger W, et al. The pharmacological profile of dienogest. Eur J Contracept Reprod Health Care. 1999;4(S1):2–13
14. Kohler G, Faustmann TA, Gerlinger C, et al. A dose-ranging study to determine the efficacy and safety of 1, 2, and 4 mg of dienogest daily for endometriosis. Int J Gynecol Obstet 2010; 108:21-5
15. Strowitzki T, Faustmann T, Gerlinger C, et al. Dienogest in the treatment of endometriosis-associated pelvic pain: a 12-week, randomized, double-blind, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol. 2010;151:193–198
16. Cosson M, Querleu D, Donnez J, et al. Dienogest is as effective as triptorelin in the treatment of endometriosis after laparoscopic surgery: Results of a prospective, multicenter, randomized study. Fertil Steril. 2002;77(4):684–692
17. Strowitzki T, Marr J, Gerlinger C, Faustmann T, Seitz C. Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: A 24-week, randomized, multicentre, open-label trial. Hum Reprod. 2010;25(3):633–641
18. Harada T, Momoeda M, Taketani Y, et al. Dienogest is as effective as intranasal buserelin acetate for the relief of pain symptoms associated with endometriosis – a randomized, double-blind, multicenter, controlled trial. Fertil Steril. 2009;91(3):675–681
19. Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol 1992;166:740-5.
20. Petraglia F, Hornung D, Seitz C, et al. Reduced pelvic pain in women with endometriosis: efficacy of long-term dienogest treatment. Arch Gynecol Obstet. 2011;285(1): 167–173
21. Momoeda M, Harada T, Terakawa N, et al. Long-term use of dienogest for the treatment of endometriosis. J Obstet Gynaecol Res. 2009;35(6):1069–1076
22. Hughes E, Brown J, Collins JJ, Farquhar C, Fedorkow DM, Vandekerckhove P. Ovulation suppression for endometriosis. Cochrane Database Syst Rev 2007 Jul18;(3):CD000155.
23. Sallam HN, Garcia-Velasco JA, Dias S, Arici A. Long-term pituitary down-regulation before in vitro fertilization (IVF) for women with endometriosis. Cochrane Database Syst Rev 2006 Jan 25;(1):CD004635
24. Moore C, Carol W, Graser T, Mellinger U, Walter F. Influence of dienogest on ovulation in young fertile women. Clin Drug Invest. 1999; 18:271–278
25. Momoeda M, Taketani Y. Randomized double-blind, multicentre, parallel-group dose–response study of dienogest in patients with endometriosis. Jpn Pharmacol Ther. 2007;35:769–783.