Pharmacological action
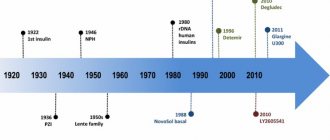
: DNA recombinant human insulin. It is a short-acting insulin preparation.
The main effect of the drug is the regulation of glucose metabolism. In addition, it has an anabolic effect. In muscle and other tissues (with the exception of the brain), insulin causes rapid intracellular transport of glucose and amino acids and accelerates protein anabolism. Insulin promotes the conversion of glucose into glycogen in the liver, inhibits gluconeogenesis, and stimulates the conversion of excess glucose into fat.
Humulin Regular solution d/i 100IU/ml 3ml N5
Humulin Regular Pharmacological action: It is a preparation of insulin identical to human (pH = 6.6-8.0). Created on the basis of recombinant DNA. Humulin has the exact amino acid sequence of human insulin. Short-acting drug. The onset of action of the drug is 30 minutes after administration. The maximum effect is between the 1st and 3rd hour. Indications for use: Insulin-dependent form of diabetes mellitus; hyperglycemic coma (loss of consciousness, characterized by a complete lack of body response to external stimuli, due to an excessive increase in blood sugar levels), when preparing a patient suffering from diabetes for surgery; for injuries or acute infections in a patient with diabetes. Directions for use: The dose of the drug is determined by the doctor in each specific case depending on the patient’s condition. When using humulin regular in its pure form, the drug is administered 3-4 times a day. Subcutaneous and intravenous administration of the drug is possible. In order to enhance the effect and prolong (extend) the time of action, you can use Humulin Regular in a mixture with Humulin M, Lente or NHP. In this case, short-acting insulin should be drawn into the syringe first. The need for humulin may increase during illness or stress, or while taking medications with glycemic activity (increasing blood sugar levels), oral contraceptives, steroids, thyroid hormones. The requirements for humulin may be reduced in case of renal or liver failure, when taken together with MAO inhibitors, beta-blockers. Side effects: Rarely - lipodystrophy (reduction in the volume of adipose tissue in the subcutaneous tissue), insulin resistance (lack of response to insulin administration), hypersensitivity reactions. Contraindications: Hypoglycemia (low blood sugar), hypersensitivity to the drug. Release form: Neutral insulin solution for injection, 10 ml in bottles; 1.5 ml cartridges for Pena Becton Dickinson. Storage conditions: List B. In a place protected from light (in the original cardboard box) at a temperature of +2 to +8 ° C. Avoid freezing. Synonyms: Insulin. Composition: 1 ml of the drug contains 40 units or 100 units. The active substance is a substance identical to human insulin (30% amorphous, 70% crystalline).
Directions for use and dosage:
The dose is determined by the doctor individually depending on the glycemic level.
The drug should be administered subcutaneously, intravenously, or possibly intramuscularly.
The drug is injected subcutaneously into the area of the shoulder, thigh, buttock or abdomen. The injection site must be alternated so that the same site is used no more than approximately 1 time per month.
When administered subcutaneously, care must be taken to avoid entering a blood vessel. After the injection, do not massage the injection site. Patients should be trained in the correct use of insulin delivery devices.
Vials of Humulin Regular do not require resuspension and can only be used if their contents are a clear, colorless liquid without visible particles.
Vials should be checked carefully. Do not use the product if it contains flakes or if hard white particles have stuck to the bottom or walls of the bottle, creating the effect of a frosty pattern.
The contents of the bottle should be drawn into an insulin syringe corresponding to the concentration of insulin administered, and the required dose of insulin should be administered as directed by the doctor.
Humulin Regular can be administered in combination with Humulin NPH. To do this, short-acting insulin should be drawn into the syringe first to prevent longer-acting insulin from entering the vial. It is advisable to administer the prepared mixture immediately after mixing. To administer the exact amount of each type of insulin, you can use a separate syringe for Humulin Regular and Humulin NPH.
You should always use an insulin syringe that matches the concentration of insulin being administered.
Humulin® Regular
The dose of Humulin® Regular is determined by the doctor individually depending on the concentration of glucose in the blood.
Humulin® Regular can be administered subcutaneously or intravenously. For intravenous administration, either an insulin syringe or an infusion pump is used. The required dose of insulin is diluted in 0.9% sodium chloride solution. The dose and rate of administration are determined in accordance with clinical recommendations for the administration of insulin in a hospital setting.
Using a syringe pen, Humulin® Regular can only be administered subcutaneously.
The temperature of the administered drug should be at room temperature.
Subcutaneous injections should be done in the area of the shoulder, thigh, buttock or abdomen. Injection sites should be rotated so that the same site is used no more than approximately once a month. When injecting insulin subcutaneously, care must be taken not to enter a blood vessel when injecting. After the injection, do not massage the injection site. Patients should be taught how to properly use a pen to administer insulin.
For the drug Humulin® Regular in cartridges
Cartridges containing Humulin® Regular do not require resuspension and can only be used if their contents are a clear, colorless solution without visible particles.
The design of the cartridges does not allow mixing their contents with other insulins directly in the cartridge itself. Cartridges are not intended to be refilled.
Cartridges can only be used with refillable injector pens manufactured by Eli Lilly. Do not use cartridges with syringe pens from other manufacturers, as in this case the required dosing accuracy of the drug will not be ensured. Before giving the injection, you must read the instructions for using a syringe pen for administering insulin.
To avoid the transmission of possible diseases, each cartridge should only be used by one patient, even when changing needles and insulin pens.
For the drug Humulin® Regular in the KwikPen™ syringe pen
Syringe pens with Humulin® Regular do not require resuspension.
Before performing an injection, you must read the Instructions for Use of the KwikPen™ syringe pen.
To avoid the transmission of possible diseases, each pen should only be used by one patient, even if needles are changed.
Instructions for using the KwikPen™ syringe pen
Humulin® Regular
100 IU/ml, 3 ml cartridge
PLEASE READ THE MANUAL BEFORE USE
Read this guide before using insulin for the first time. KwikPen™ syringe pens, you must read the manual again, because... it may contain updated information. The information contained in this manual does not replace a conversation with your doctor about the disease and your treatment.
QuickPen ™ (“pen”) is a disposable, prefilled pen containing 300 units of insulin. With one pen you can inject several doses of insulin. Using this syringe pen, you can administer a dose with an accuracy of 1 unit. You can administer from 1 to 60 units per injection. If your dose is more than 60 units, you will need to give more than one injection. With each injection, the piston moves only slightly, and you may not notice a change in its position. The plunger will only reach the bottom of the cartridge when you have used up all 300 units contained in the pen.
The syringe pen cannot be shared with other people, even when using a new needle. Do not reuse needles. Do not share needles with other people. The needle can transmit infection, which can lead to infection.
It is not recommended for use in patients with low vision or complete loss of vision without the assistance of well-visioned people trained in the correct use of the pen.
To perform the injection you need:
KwikPen™ syringe pen with insulin.
QuickPen™ pen (Becton, Dickinson and Company (BD) pen needles are recommended).
- A swab soaked in alcohol.
Preparing a syringe pen for insulin administration:
— Wash your hands with soap.
— Check your pen to make sure it contains the type of insulin you need. This is especially important if you use more than 1 type of insulin.
— Do not use pens if the expiration date stated on the label has expired.
— Use a new needle with each injection to prevent infections and to avoid needles becoming clogged.
Stage 1:
— Remove the cap of the syringe pen.
— Do not remove the label of the syringe pen.
— Wipe the rubber disc with a swab soaked in alcohol.
Stage 2:
— Check the appearance of the insulin.
HUMULIN® Regular should be transparent and colorless. Do not use if it is cloudy, discolored, or contains particles or clumps.
| Stage 3: — Take a new needle. — Remove the paper sticker from the outer needle cap. Stage 4: — Place the cap with the needle directly on the syringe pen and screw it tightly. |
Stage 5:
— Remove the outer needle cap. Don't throw it away.
— Remove the inner needle cap and discard it.
Checking the syringe pen for drug intake
This check should be carried out before each injection.
— Checking the syringe pen for the supply of the drug is carried out to remove air from the needle and cartridge, which may accumulate during normal storage, and to ensure the proper operation of the syringe pen.
- If do not do this check before each injection, you may give either too low or too high a dose of insulin.
| Stage 6: — To check the syringe pen for drug delivery, set 2 units by rotating the dose button. Stage 7: — Hold the pen with the needle pointing up. Lightly tap the cartridge holder to collect air bubbles at the top. Stage 8: — Continue holding the pen with the needle pointing up. Press the dose button until it stops and an “O” appears in the dose indicator window. While holding the dose button, slowly count to 5. — You should see insulin at the tip of the needle. — If a drop of insulin does not appear at the tip of the needle, repeat the steps of checking the syringe pen for drug delivery. The check can be carried out no more than 4 times. — If insulin still does not appear, change the needle and repeat checking the syringe pen for drug delivery. The presence of small air bubbles is normal and does not affect the administered dose. |
Dose selection
- You can administer from 1 to 60 units per injection.
— If your dose is more than 60 units, you will need to give more than one injection.
— If you need help with how to divide the dose correctly, contact your doctor.
— For each injection, you should use a new needle and repeat the procedure for checking the syringe pen for drug delivery.
| Stage 9: — To dial the dose of insulin you need, turn dose button. The dose indicator should be in line with the number of units corresponding to your dose. — With one turn, the dose button moves by 1 unit. — Each time you turn the dose button, a click is heard. - Do NOT select the dose by counting the clicks as this may result in the wrong dose being dialed. — The dose can be adjusted by turning the dose button in the desired direction until the number corresponding to your dose appears in the dose indicator window in line with the dose indicator. — Even numbers are indicated on the scale. — Odd numbers, after the number 1, are indicated by solid lines. — Always check the number in the dose indicator window to make sure you are taking the correct dose. |
— If there is less insulin left in the syringe pen than you need, you will not be able to administer the dose you need using this syringe pen.
— If you need to inject more units than are left in the pen, you can:
— inject the amount remaining in your pen, and then use a new pen to administer the rest of the dose, or
- take a new syringe pen and inject the full dose.
— There may be a small amount of insulin left in the pen that you cannot inject.
Carrying out the injection
— Inject insulin strictly in accordance with what your doctor has indicated.
- Change (alternate) injection sites with each injection.
- Do not try to change the dose during the injection.
| Stage 10: — Choose the injection site. Insulin is injected under the skin (subcutaneously) into the anterior abdominal wall, buttocks, thighs, or shoulders. — Wipe the skin with a swab soaked in alcohol and wait until the alcohol dries. Step 11: - Insert the needle under the skin. — Press the dose button all the way. -Hold down the dose button and slowly count to 5, then remove the needle from the skin. Do not attempt to inject insulin by turning the dose button. When you rotate the dose button, insulin NOT flow. |
Stage 12:
Remove the needle from the skin.
It is normal for a drop of insulin to remain on the tip of the needle. This does not affect the accuracy of your dose.
Check the number in the dose indicator window.
If the dose indicator window shows “0”, it means that you have administered the dialed dose in full.
If you do not see a “0” in the dose indicator window, you should not refill the dose. Insert the needle under the skin again and complete the injection.
If you still think that the dose you have taken has not been administered in full, do not inject again. Check your blood glucose levels and act as directed by your doctor. If you need 2 injections to give the full dose, be sure to give the second injection.
With each injection, the piston moves only slightly, and you may not notice a change in its position.
If you notice a drop of blood after removing the needle from the skin, gently press a clean gauze pad onto the injection site. Do not rub this area.
After injection
| Stage 13: — Carefully put on the outer needle cap. Stage 14: - Unscrew the needle and cap and discard it, as described below (see section “Disposal of syringe pens and needles”). — Do not store a pen with a needle attached. avoiding insulin leakage, needle clogging and air getting into the syringe pen. Stage 15: — Place the cap on the syringe pen, aligning the clamp cap with a dose indicator and pressing it. |
Disposal of syringe pens and needles
— Place used needles in a sharps container or hard plastic container with a tight-fitting lid. Do not dispose of needles in areas designated for household waste.
— The used syringe pen can be thrown away with household waste after removing the needle.
— Ask your healthcare professional about how to dispose of your sharps container.
— The needle disposal guidelines provided in this manual do not replace the rules, regulations, or policies of each healthcare facility.
Storing a syringe pen
Unused syringe pens
— Store unused pens in the refrigerator at a temperature between 2°C and 8°C.
— Do not freeze the insulin you use. If it has been frozen, do not use it.
— Unused pens can be stored until the expiration date stated on the label if stored in the refrigerator.
Syringe pen currently in use
— Store the pen you are currently using at room temperature between 15°C and 25°C, protected from heat and light.
— When the expiration date indicated on the package expires, the used syringe pen must be thrown away, even if there is insulin left in it.
General information about the safe and effective use of pen syringes
— Keep the pen and needles out of the reach of children.
— Do not use the pen if any part of it appears broken or damaged.
— Always carry a spare pen with you in case your pen gets lost or broken.
Troubleshooting
— If you cannot remove the cap from the syringe pen, carefully twist it back and forth, and then pull the cap.
— If the dose dial button is difficult to press:
— Press the dose dial button more slowly. By slowly pressing the dose dial button it is easier to inject.
— The needle may be blocked. Insert a new needle and check the syringe pen for drug delivery.
— Dust or other substances may have gotten inside the syringe pen. Throw away this syringe pen and get a new one.
Special instructions:
Transferring a patient to another type of insulin or to an insulin preparation with a different trade name should occur under strict medical supervision. Changes in insulin activity, insulin type (eg, M3, NPH), type (porcine, human insulin, human insulin analog), or production method (DNA recombinant insulin or animal insulin) may require dosage adjustments.
The need for dose adjustment may be required as early as the first administration of a human insulin product after an animal insulin product or gradually over several weeks or months after the transfer.
Impact on the ability to drive vehicles and operate machinery: during hypoglycemia, the patient’s ability to concentrate may deteriorate and the speed of psychomotor reactions may decrease. This can be dangerous in situations where these abilities are especially needed (driving a car or operating machinery). Patients should be advised to take precautions to avoid hypoglycemia while driving. This is especially important for patients with mild or absent warning signs of hypoglycemia or who frequently develop hypoglycemia. In such cases, the doctor should evaluate the advisability of the patient driving a car.
Humulin Regular, 100 IU/ml, solution for injection, 10 ml, 1 pc.
PC,
in the area of the shoulder, thigh, buttock or abdomen. Possible intramuscular and intravenous administration.
The dose of Humulin® Regular is determined by the doctor individually depending on the concentration of glucose in the blood.
The temperature of the administered drug should be at room temperature. Injection sites should be rotated so that the same site is used no more than approximately once a month. When administering insulin subcutaneously, care must be taken not to enter a blood vessel. After the injection, do not massage the injection site.
Patients should be trained in the correct use of the insulin delivery device. The insulin administration regimen is individual.
Humulin® Regular can be administered in combination with Humulin® NPH (see instructions for mixing insulins).
Preparing for the introduction
For the drug Humulin® Regular in bottles.
Vials containing Humulin® Regular do not require resuspension and can only be used if their contents are a clear, colorless solution without visible particles. You should always use an insulin syringe that matches the concentration of insulin being administered.
For the drug Humulin® Regular in cartridges.
Cartridges containing Humulin® Regular do not require resuspension and can only be used if their contents are a clear, colorless solution without visible particles. The design of the cartridges does not allow mixing their contents with other insulins directly in the cartridge itself. Cartridges are not intended to be refilled. Before giving the injection, you must read the manufacturer's instructions for using a syringe pen for administering insulin.
For the drug Humulin® Regular in the KwikPen™ syringe pen.
Before performing an injection, you must read the Instructions for Use of the KwikPen™ syringe pen.
Instructions for using the KwikPen™ syringe pen
The KwikPen™ syringe pen is easy to use. It is a device for administering insulin (“insulin syringe pen”) containing 3 ml (300 units) of an insulin preparation with an activity of 100 IU/ml. You can administer from 1 to 60 units of insulin per injection. You can set the dose with an accuracy of one unit. If too many units are set, the dose can be adjusted without losing insulin. The QuickPen™ syringe pen is recommended for use with needles manufactured by Becton, Dickinson and Company (BD)
for syringe pens. Before using the pen, you must ensure that the needle is completely attached to the pen.
In the future, the following rules should be followed.
1. Follow the rules of asepsis and antisepsis recommended by the attending physician.
2. Wash your hands.
3. Select the injection site.
4. Wipe the skin at the injection site.
5. Rotate injection sites so that the same site is used no more than approximately once a month.
Preparation of the KwikPen™ syringe pen and administration
1. Pull the pen cap to remove it. Do not rotate the cap. Do not remove the label from the syringe pen. Make sure the insulin is checked for insulin type; expiration date; appearance.
2. Take a new needle. Remove the paper sticker from the outer needle cap. Use an alcohol swab to wipe the rubber disc at the end of the cartridge holder. Place the needle in the cap straight along the axis onto the syringe pen. Screw the needle until completely connected.
3. Remove the outer cap from the needle. Don't throw it away. Remove the inner cap from the needle and discard it.
4. Check the KwikPen™ syringe pen for insulin supply. You should check your insulin supply every time. Checking the delivery of insulin from the pen should be performed before each injection until a stream of insulin appears to ensure that the pen is ready to administer a dose.
If you do not check your insulin before the trickle appears, you may receive too little or too much insulin.
5. Fix the skin by stretching it or gathering it into a large fold. Insert the needle subcutaneously using the injection technique recommended by the attending physician. Place your thumb on the dose button and press firmly until it stops completely. To administer a full dose, hold the dose button and slowly count to 5.
6. Remove the needle and gently press the injection site with a cotton swab for a few seconds. Do not rub the injection site. If insulin drips from the needle, the patient most likely did not hold the needle under the skin long enough. It is normal to have a drop of insulin at the tip of the needle and will not affect your dose.
7. Using the needle protective cap, unscrew the needle and discard it.
Even numbers are printed in the dose indicator window as numbers, odd numbers are printed as straight lines between even numbers.
If the dose required to deliver exceeds the number of units remaining in the cartridge, you can inject the remaining amount of insulin in the pen and then use a new pen to complete the required dose, or administer the entire dose required using a new pen.
Do not attempt to inject insulin by rotating the dose button. The patient will not receive insulin if he rotates the dose button. It is necessary to press the dose button along a straight axis in order to receive a dose of insulin.
Do not try to change the insulin dose during the injection.
Note.
The pen will not allow the patient to set an insulin dose greater than the number of units remaining in the pen. If you are not sure that the full dose has been administered, do not administer another one. You should read and follow the instructions contained in the instructions for use of the drug. The label on the pen should be checked before each injection to ensure that the drug has not expired and that the patient is using the correct type of insulin; Do not remove the label from the syringe pen.
The color of the dose button of the QuickPen™ syringe pen corresponds to the color of the stripe on the label of the syringe pen and depends on the type of insulin. In this manual, the dose button is shown in gray. The beige color of the body of the QuickPen™ syringe pen indicates that it is intended for use with the Humulin® line of drugs.
Storage and disposal
The syringe pen cannot be used if it has been out of the refrigerator for more than the time specified in the instructions for use.
Do not store a pen with a needle attached to it. If the needle is left attached, insulin may leak out of the pen, the insulin may dry out inside the needle, causing the needle to become clogged, or air bubbles may form inside the cartridge.
Syringe pens that are not in use should be stored in the refrigerator at a temperature of 2 to 8 °C. Do not use the pen if it has been frozen.
The pen currently in use should be stored at room temperature, away from heat and light, and out of the reach of children.
Dispose of used needles in a puncture-resistant, lockable container (such as a biohazard or waste container), or as directed by your healthcare provider.
The needle must be removed after each injection.
Dispose of used pens without needles attached as directed by your healthcare provider and in accordance with local medical waste disposal regulations.
Do not recycle a filled sharps container.