Humalog®
Dosage regimen
The dose of Humalog® is determined by the doctor individually depending on the concentration of glucose in the blood. The insulin administration regimen is individual.
Humalog® can be administered shortly before meals. If necessary, Humalog® can be administered shortly after meals.
When administered subcutaneously, Humalog® begins to act quickly and has a shorter duration of action (from 2 to 5 hours) compared to soluble human insulin. The rapid onset of action allows Humalog® to be administered close to a meal. The time course of action of any insulin can vary significantly between patients or at different times within the same patient. The faster onset of action compared to soluble human insulin is maintained regardless of injection site. The duration of action of Humalog® depends on the dose, injection site, blood supply, temperature and physical activity.
The drug Humalog®, on the recommendation of a doctor, can be used in combination with longer-acting insulin.
The drug Humalog® 100 IU/ml in the KwikPen® syringe pen and the drug Humalog® 200 IU/ml in the KwikPen® syringe pen
Humalog® in the KwikPen® syringe pen is available in 2 concentrations: Humalog® 100 IU/ml and Humalog® 200 IU/ml. Both prefilled pens, Humalog® 100 IU/ml and Humalog® 200 IU/ml, allow you to administer from 1 to 60 IU in 1 IU increments per injection. When transferring a patient from one drug concentration to another, dose recalculation is not required.
The number of collected MEs for both concentrations of the drug is indicated in the dose indicator window of the KwikPen® syringe pen.
The drug Humalog® 200 IU/ml in the KwikPen® syringe pen should be used for the treatment of patients with diabetes mellitus who require a daily dose of short-acting insulin of more than 20 IU. A cartridge with a solution of insulin lispro with a concentration of 200 IU/ml cannot be removed from a syringe pen (KwikPen®) or mixed with other insulins.
Special patient groups
Kidney failure
In renal failure, the need for insulin may be reduced.
Liver failure
In patients with liver failure, insulin requirements may be reduced as a result of decreased gluconeogenesis and insulin metabolism; however, in patients with chronic liver failure, increased insulin resistance may lead to increased insulin requirements.
Mode of application
Humalog® 200 IU/ml should be administered subcutaneously.
Subcutaneous injections should be given in the upper arm, thigh, buttock or abdomen. Injection sites must be alternated so that the same site is used no more than approximately once a month.
When administering Humalog® subcutaneously, care must be taken to avoid the drug entering a blood vessel. After the injection, do not massage the injection site. Patients should be taught proper injection technique.
Humalog® 200 IU/ml in the KwikPen® syringe pen is not intended for administration using an insulin pump.
The drug Humalog® 200 IU/ml in the KwikPen® syringe pen is not intended for intravenous administration.
INSTRUCTIONS FOR USE OF THE QUIKPEN® PEN
UMALOG® QUIKPEN® 200 IU/ml, 3 ml
PLEASE READ THIS MANUAL BEFORE USING
USE ONLY IN THIS PEN TO AVOID SEVERE OVERDOSE
Read the QuickPen® Instructions for Use before using Humalog®, 200 IU/ml. Read the manual each time you start using your new KwikPen® Humalog® 200 IU/ml as there may be new information. The information contained in the QuickPen® manual is not intended to replace consultation with a physician regarding your medical condition or treatment.
Humalog® QuickPen®, 200 IU/mL (“pen”) is a single-use, prefilled pen that contains 3 mL (600 units, 200 units/mL) of Humalog®. With one pen you can inject several doses of insulin. The dose of the drug can be set with an accuracy of 1 unit. In one injection you can inject from 1 to 60 units. If your dose is more than 60 units, you will need to give more than one injection.
With each injection, the piston moves only slightly, and you may not notice a change in its position. The plunger will only reach the bottom of the cartridge when you have used up all 600 units contained in the pen.
This pen is designed to deliver larger doses than other pens you may have previously used. Set your usual dose as directed by your doctor.
Humalog® QuickPen® is available in two dosages: 100 IU/ml and 200 IU/ml. Administer Humalog® 200 IU/ml using your pen ONLY. DO NOT transfer insulin from your pen to another insulin delivery device. Syringes will not provide an accurate dose of 200 IU/ml insulin. This may cause a severe overdose, which will result in very low blood glucose concentrations and may be life-threatening.
The syringe pen cannot be shared with other people, even when using a new needle. Do not reuse needles. Do not share needles with other people. The needle can transmit infection, which can lead to infection.
The pen is not recommended for use by patients with low vision or complete loss of vision without the assistance of people trained in the proper use of the pen.
How to recognize the drug Humalog® 200 IU/ml in the KwikPen® syringe pen
| Syringe pen color: | Dark grey. |
| Dose button: | Dark gray with a burgundy ring at the end. |
| Label: | Dosage "200 IU/ml" burgundy color on a white background. Yellow warning on the cartridge holder. |
To perform the injection it is necessary
— Humalog®, 200 IU/ml, in a KwikPen® syringe pen;
— A needle compatible with the KwikPen® syringe pen (it is recommended to use needles manufactured by BD [Becton, Dickinson and Company]);
- Alcohol wipe.
Needles and alcohol wipes are not included.
Preparing a syringe pen for insulin injection
— Wash your hands with soap.
— Check your pen to make sure it contains the type of insulin you need. This is especially important if you are using more than one type of insulin.
— Do not use pens that have expired, which is indicated on the label. Do not use the pen for more than 28 days from the date of use.
— Use a new needle with each injection to prevent infection and to avoid needle clogging.
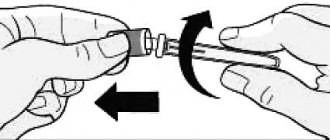
| Stage 1: Pull and remove the pen cap. — Do not remove the label on the pen. Wipe the rubber disc with an alcohol wipe. The drug should be transparent and colorless. Do not use the product if the solution is cloudy, colored, or contains particles or clots. Stage 2: Get a new needle. Remove the paper sticker from the outer needle cap. Stage 3: Place the cap with the needle directly on the syringe pen and screw it tightly. Stage 4: Remove the outer needle cap. Don't throw it away. Remove the inner needle cap and discard it. |
Checking the syringe pen for drug intake
This check should be carried out before each injection.
— Checking the syringe pen for the supply of the drug is carried out to remove air from the needle and cartridge, which may accumulate during normal storage, and to ensure the proper operation of the syringe pen.
- If you do not do this check before each injection, you may give either too low or too high a dose of insulin.
| Stage 5: To check the syringe pen for drug delivery, set 2 units by rotating the dose button. |
| Stage 6: Hold the pen with the needle pointing up. Lightly tap the cartridge holder to collect air bubbles at the top. |
| Stage 7: Continue holding the pen with the needle pointing up. Press the dose button until an “O” appears in the dose indicator window. While holding the dose button, slowly count to 5. Insulin should appear at the tip of the needle. ■ If a drop of insulin does not appear at the tip of the needle, repeat the steps of checking the pen for drug delivery. The check can be carried out no more than 8 times. ■ If insulin still does not appear, change the needle and test the drug again. The presence of small air bubbles is normal and does not affect the administered dose. |
Dose selection
This pen has been designed to deliver the dose indicated in the window
dose indicator. Set your usual dose as directed by your doctor.
- You can administer from 1 to 60 units per injection.
- If your dose is greater than 60 units, you will need to take more than one
injections.
— If you need help with how to divide the dose correctly, contact your doctor.
— For each injection, use a new needle and repeat the procedure
checking the syringe pen for the supply of the drug.
| Stage 8: To dial the dose of insulin you need, turn the dose button. The dose indicator should be in line with the number of units corresponding to your dose. — With one turn, the dose button moves by 1 unit. — Each time you turn the dose button, a click is heard. - Do NOT select the dose by counting the clicks as this may result in the wrong dose being dialed. — The dose can be adjusted by turning the dose button in the desired direction until the number corresponding to your dose appears in the dose indicator window in line with the dose indicator. — Even numbers are indicated on the scale. — Odd numbers, after the number 1, are indicated by solid lines. Always check the number in the dose indicator window to make sure you are taking the correct dose. |
— If there is less insulin left in the syringe pen than you need, you will not be able to administer the dose you need using this syringe pen.
— If you need a dose that exceeds the number of units remaining in the pen, you can:
— inject the amount remaining in your pen and then use a new pen to administer the rest of the dose, or
- take a new syringe pen and inject the full dose.
— There may be a small amount of insulin left in the pen that you cannot inject. Do not transfer this insulin to another syringe. This can lead to a significant overdose.
Carrying out the injection
— Carry out the insulin injection strictly in accordance with what your doctor has shown.
- Change (alternate) injection sites with each injection.
- Do not try to change the dose during the injection.
| Stage 9: Select the injection site. Humalog®, 200 IU/ml is injected subcutaneously into the abdomen, buttocks, thighs or upper arms. Clean your skin with an alcohol wipe before injecting. Stage 10: Insert the needle into the skin. Press the dose button all the way down. Hold down the dose button and slowly count to 5, then remove the needle from your skin. Do not attempt to inject insulin by turning the dose button. When you rotate the dose button, insulin does NOT flow. Stage 11: Remove the needle from the skin. — It is normal if there is a drop of insulin left on the tip of the needle. This will not affect the accuracy of your dose. Check the number in the dose indicator window. — If you see “0” in the dose indicator window, it means that you have administered the dialed dose in full. — If you do not see “0” in the dose indicator window, do not set the dose again. Insert the needle under the skin again and complete the injection. — If you still think that the dose you have taken has not been administered in full, do not inject again. Check your blood glucose levels and act as directed by your doctor. |
With each injection, the piston moves only slightly, and you may not notice a change in its position.
If you notice a drop of blood after removing the needle from the skin, gently press a clean gauze pad onto the injection site. Do not rub this area.
After the injection
| Stage 12: Carefully replace the outer needle cap. Stage 13: Unscrew the needle and cap and dispose of it as described below (see section “Disposal of syringe pens and needles”). Do not store the pen with a needle attached to prevent insulin from leaking, clogging the needle, and causing air to enter the pen. Stage 14: Place the cap on the pen, aligning the cap clip with the dose indicator, and press the cap |
Disposal of syringe pens and needles
Place used needles in a sharps container or hard plastic container with a tight-fitting lid. Do not dispose of needles in areas designated for household waste.
The used syringe pen can be disposed of as household waste after the needle is removed.
Ask your healthcare professional about how to dispose of your sharps container.
The needle disposal guidelines provided in this manual do not replace the rules, regulations, or policies of each healthcare facility.
Storing a syringe pen
Unused syringe pens
— Store unused pens in the refrigerator at a temperature between 2°C and 8°C.
— Do not freeze the insulin you use. If it has been frozen, do not use it.
— Unused pens can be stored until the expiration date stated on the label if stored in the refrigerator.
Used syringe pen
— Do not store the pen you are currently using in the refrigerator. A syringe pen in use should be stored at a temperature not exceeding 30°C in a place protected from heat and light.
— You should throw away a pen that has been in use for more than 28 days, even if there is insulin left in it.
General information about the safe and effective use of pen syringes
— Keep the pen and needles out of the reach of children.
-Do not use the pen if any part appears broken or damaged.
— Always carry a spare pen with you in case your pen gets lost or broken.
Troubleshooting
— If you cannot remove the cap from the syringe pen, carefully twist it back and forth, and then pull the cap.
— If the dose dial button is difficult to press:
— Press the dose dial button more slowly. By slowly pressing the dose dial button it is easier to inject.
— The needle may be blocked. Put on a new needle and check the syringe pen for drug delivery.
— Dust, food or other substances may have gotten inside the syringe pen. Throw away this syringe pen and get a new one.
— Do not transfer insulin from a pen to another syringe. This can lead to severe overdose.
If you have any questions or problems regarding the use of the drug Humalog® 200 IU/ml in the KwikPen® syringe pen, contact your doctor for help or contact the Representative Office of Eli Lilly Vostok S.A. in Moscow.
Humalog® and Humalog® QuickPen® are trademarks.
Side effects of Humalog
Possible allergic reactions, reactions at the injection site, lipodystrophy, itching, rash, hypoglycemia. Hypoglycemia is the most common side effect of insulin therapy in patients with diabetes. Severe hypoglycemia can lead to loss of consciousness and, in exceptional cases, death. Patients may develop allergic reactions in the form of hyperemia, swelling or itching at the injection site. These reactions usually disappear within a few days or weeks. In some cases, these reactions may be caused by reasons not directly related to the administration of insulin (for example, skin irritation after treatment with an antiseptic or incorrect injection technique). Systemic allergic reactions caused by insulin occur much less frequently, but are potentially more dangerous. Generalized allergic reactions to insulin administration may include itching all over the body, difficulty breathing, shortness of breath, decreased blood pressure, tachycardia, or sweating. Severe cases of generalized allergic reactions can be life-threatening. The development of an acute allergic reaction requires emergency desensitizing therapy and drug replacement.
Humalog drug interactions
The need for insulin may increase with the simultaneous use of drugs with hyperglycemic effects, such as oral contraceptives, corticosteroids or thyroid hormone preparations. Insulin requirements may be reduced when taking medications that have a hypoglycemic effect, such as oral hypoglycemic drugs, salicylates (for example, acetylsalicylic acid), sulfonamides and some antidepressants (MAO inhibitors). In patients with type II diabetes mellitus, with combination therapy with a sulfonylurea and insulin lispro, the level of glycosylated hemoglobin decreases more significantly than with monotherapy with a sulfonylurea.
Humalog overdose, symptoms and treatment
Leads to the development of hypoglycemia, which is manifested by lethargy, apathy, impaired consciousness, tremor, sweating, tachycardia, vomiting and headache. Hypoglycemia can develop as a result of an overdose of insulin lispro relative to the amount of food taken, increased energy expenditure, or both. Hypoglycemia can usually be corrected by oral glucose. Dose, diet, or exercise adjustments may be necessary. More severe episodes of hypoglycemia attacks, which are accompanied by coma, convulsions or other neurological disorders, are stopped by intramuscular or subcutaneous administration of glucagon or intravenous administration of concentrated glucose solution. Carbohydrate maintenance and medical monitoring may be necessary as hypoglycemia may recur after apparent clinical improvement.
special instructions
To date, no adverse effects of insulin lispro on pregnancy or the condition of the fetus and newborn have been identified.
The goal of insulin therapy during pregnancy is to maintain adequate glucose control. Insulin requirements usually decrease in the first trimester and increase in the second and third trimesters of pregnancy. During and immediately after childbirth, the need for insulin may decrease dramatically.
Women of childbearing age with diabetes should inform their doctor about an existing or planned pregnancy.
In patients with diabetes mellitus, adjustment of the insulin dose and/or diet may be required during breastfeeding.
Transferring a patient to another type or drug of insulin with a different trade name should occur under strict medical supervision. Changes in potency, brand (manufacturer), type (eg, Regular, NPH), species (animal, human, human insulin analogue) and/or production method (DNA recombinant insulin or animal insulin) may require dosage adjustments .
The use of inadequate doses or discontinuation of treatment, especially in patients with insulin-dependent diabetes mellitus, can lead to hyperglycemia and diabetic ketoacidosis (conditions that are potentially life-threatening to the patient).
The need for insulin may be reduced if there is insufficiency of the adrenal glands, pituitary gland or thyroid gland, or with renal or liver failure. With some illnesses or emotional stress, the need for insulin may increase. Insulin dosage adjustments may also be necessary if you increase your physical activity or change your usual diet.
During hypoglycemia, the patient may experience a decrease in concentration and speed of psychomotor reactions. This can be dangerous in situations where these abilities are particularly needed (for example, driving a car or operating machinery).
Patients should be advised to take precautions to avoid hypoglycemia while driving vehicles and operating machinery. This is especially important for patients with mild or absent warning signs of hypoglycemia or those with frequent hypoglycemia. In such cases, the doctor must evaluate the appropriateness of the patient driving a car and operating machinery.
pharmachologic effect
Hypoglycemic drug, a combination of insulin analogues with rapid and medium duration of action. Humalog® Mix 25 is a DNA - recombinant analogue of human insulin and is a ready-made mixture consisting of a solution of insulin lispro (a fast-acting analogue of human insulin) and a suspension of insulin lispro protamine (an analogue of human insulin with an average duration of action).
The main effect of insulin lispro is the regulation of glucose metabolism. In addition, it has anabolic and anti-catabolic effects on various tissues of the body. In muscle tissue, there is an increase in the content of glycogen, fatty acids, glycerol, increased protein synthesis and an increase in amino acid consumption, but at the same time there is a decrease in glycogenolysis, gluconeogenesis, ketogenesis, lipolysis, protein catabolism and amino acid release.
Insulin lispro has been shown to be equimolar to human insulin, but its action is faster and lasts for a shorter time. The onset of action of the drug is approximately 15 minutes, which allows it to be administered immediately before meals (0-15 minutes before meals), compared to regular human insulin. After subcutaneous injection of Humalog Mix 25, a rapid onset of action and early peak activity of insulin lispro are observed. The action profile of insulin lispro protamine is similar to that of regular isophane insulin, with a duration of action of approximately 15 hours.