Plegridy solution for subcutaneous administration 125 mcg No. 2 syringe pen
Content
Indications for use Contraindications Use during pregnancy and breastfeeding Method of administration and dosage Use in special groups of patients Side effects Overdose Interaction with other drugs Effect on the ability to drive a car and operate machinery Storage conditions Shelf life
Indications for use
Treatment of relapsing-remitting multiple sclerosis in adults.
Contraindications
- Hypersensitivity to natural or recombinant interferon beta or peginterferon or any excipient of this drug.
- Initiation of therapy during pregnancy.
- Severe depression and/or suicidal thoughts.
- Children under 18 years of age.
Use during pregnancy and lactation
Women with preserved childbearing potential
Women of childbearing potential should use reliable methods of contraception. If a patient becomes pregnant or plans to become pregnant while using Plegridy, she should be informed of the potential risks; discontinuation of therapy should also be considered. In women with a high pre-treatment relapse rate, the risk of significant relapse after discontinuation of Plegridy therapy due to pregnancy should be weighed against the increased risk of spontaneous abortion during treatment.
Pregnancy
Data on the use of Plegridy in pregnant women are limited. The findings indicate an increased risk of spontaneous abortion. It is contraindicated to start treatment with Plegridy during pregnancy.
Breastfeeding period
It has not been established whether peginterferon beta-1a is excreted in human breast milk. Given the possibility of serious adverse reactions in the newborn, it is necessary to stop either breastfeeding or treatment with Plegridy.
Fertility
There are no data on the effects of peginterferon beta-1a on human fertility. In animals, anovulatory effects were observed when the drug was administered in very high doses. Data on the effect of peginterferon beta-1a on male fertility are also lacking.
Directions for use and doses
The use of Plegridy should be initiated under the supervision of a physician experienced in the treatment of multiple sclerosis.
The effectiveness of Plegridy was demonstrated in comparison with placebo. There are no data on the effectiveness of Plegridy compared with non-pegylated interferon beta, or in patients switched to Plegridy after treatment with non-pegylated interferon beta. This must be taken into account when changing pegylated interferon to non-pegylated interferon, and vice versa.
Dosing
The recommended therapeutic dose of Plegridy is 125 mcg, administered subcutaneously once every 2 weeks (14 days).
Start of therapy
Treatment is recommended to begin with a dose of 63 mcg (Dose 1, Day 1), then increase to 94 mcg (Dose 2, Day 14) and reach the full dose of 125 mcg (Dose 3, Day 28). Then every
weeks (14 days), it is recommended to administer the full dose (125 mcg), see Table 2. The initial treatment package contains the first two dosages (63 mcg and 94 mcg).
A gradual increase in dose at the beginning of treatment contributes to better tolerability of flu-like symptoms that accompany the initiation of interferon use. Prophylactic and concomitant use of anti-inflammatory, analgesic, and/or antipyretic agents may prevent or alleviate the flu-like symptoms that sometimes accompany interferon treatment.
If a dose of Plegridy is accidentally missed, it should be administered as quickly as possible according to the following recommendations:
If there are 7 days or more until your next scheduled dose:
Give the missed dose immediately. The subsequent dose is administered as planned.
If there are less than 7 days left until your next scheduled dose:
A new schedule of scheduled injections (once every 2 weeks) should begin on the day of the missed dose. Plegridy should not be administered more frequently than every 7 days.
Use in special patient groups
Elderly patients
In patients over the age of 65 years, the safety and effectiveness of Plegridy have not been sufficiently studied due to the limited number of patients in this age group included in clinical trials.
Kidney failure
According to clinical studies in patients with mild, moderate, severe renal failure, as well as end-stage renal failure, no dose adjustment of Plegridy is required.
Liver failure
Plegridy has not been studied in patients with hepatic impairment.
Children
The safety and effectiveness of Plegridy have not been studied in patients under 18 years of age, so there are no data on the use of the drug in this age group.
Method of administration
Plegridy is intended for subcutaneous administration.
It is recommended that the patient be taught the correct technique for subcutaneous injections using a prefilled syringe or pen. Patients should be advised to change injection sites. The drug is usually injected under the skin of the abdomen, shoulder or thigh.
Each prefilled syringe or pen comes with a needle. Prefilled syringes and pens are for single use only and should be discarded after use.
Precautions before using the drug.
Before injection, the Plegridy drug removed from the refrigerator must be warmed under natural conditions to room temperature (about 30 minutes). The use of external heating sources such as hot water is prohibited.
Within the specified shelf life and in the absence of a refrigerator, the drug can be stored at temperatures up to 25 ° C in a place protected from light for no more than 30 days (see section “Shelf life”).
Prefilled syringes and pens should not be used if the liquid they contain is colored, cloudy, contains visible particles, or has been frozen. The liquid in the syringe or pen should be transparent, colorless or slightly yellow.
Side effect
- Blood and lymphatic system disorders: thrombocytopenia, thrombotic microangiopathy, including thrombotic thrombocytopenic purpura/hemolytic uremic syndrome;
- Immune system disorders: hypersensitivity reaction;
- Nervous system disorders: headache, seizures;
- Disorders of the respiratory system, chest and mediastinal organs, pulmonary arterial hypertension;
- Gastrointestinal disorders: nausea, vomiting;
- Skin and subcutaneous tissue disorders: itching, urticaria;
- Musculoskeletal and connective tissue disorders: myalgia, arthralgia;
- Renal and urinary tract disorders: nephrotic syndrome, glomerulosclerosis;
- General disorders and disorders at the injection site, erythema at the injection site, flu-like syndrome, fever, chills, pain at the injection site, asthenia, itching at the injection site, hyperthermia, pain, swelling at the injection site, warmth at the injection site, hematoma at the injection site injections, rashes at the injection site, swelling at the injection site, change in skin color at the injection site, inflammation at the injection site, necrosis at the injection site;
- Laboratory and instrumental data: increased body temperature, increased activity of alanine aminotransferase, increased activity of aspartate aminotransferase, increased activity of gamma-glutamyltransferase, decreased hemoglobin concentration, decreased number of leukocytes, decreased number of platelets.
- Mental disorders: depression.
Overdose
There have been no cases of overdose of Plegridy. However, in case of overdose, appropriate supportive therapy is recommended.
Interaction with other drugs
Interaction studies between Plegridy and other drugs have not been conducted. According to clinical data, patients with multiple sclerosis can receive combination therapy with Plegridy and glucocorticosteroids during disease relapses. Interferons decreased the activity of liver cytochrome P450 enzymes in humans and animals. Plegridy should be used with caution in combination with drugs with a narrow therapeutic index, in which clearance is significantly dependent on the hepatic cytochrome P450 system, for example, with some classes of antiepileptic drugs and antidepressants.
Impact on the ability to drive a car and operate machinery
Adverse reactions from the central nervous system (for example, nausea) can affect the ability to drive a car and perform potentially dangerous activities that require increased concentration and speed of psychomotor reactions.
Storage conditions
Store at a temperature of 2 to 8 C, protected from light. Do not freeze.
Keep out of the reach of children.
Best before date
3 years.
Within the specified shelf life and in the absence of a refrigerator, the drug can be stored at temperatures up to 25 ° C in a place protected from light for no more than 30 days.
Do not use after the expiration date stated on the package.
Plegridy
The use of Plegridy should be initiated under the supervision of a physician experienced in the treatment of multiple sclerosis.
The effectiveness of Plegridy was demonstrated in comparison with placebo. There are no data on the effectiveness of Plegridy compared with non-pegylated interferon beta, or in patients switched to Plegridy after treatment with non-pegylated interferon beta. This must be taken into account when changing pegylated interferon to non-pegylated interferon, and vice versa (see section “Pharmacological properties”).
Dosing
The recommended therapeutic dose of Plegridy is 125 mcg, administered subcutaneously once every 2 weeks (14 days).
Start of therapy
Treatment is recommended to begin with a dose of 63 mcg (Dose 1, Day 1), then increase to 94 mcg (Dose 2, Day 14) and reach the full dose of 125 mcg (Dose 3, Day 28). Thereafter, the full dose (125 mcg) is recommended every 2 weeks (14 days), see Table 2.
The package for starting a course of treatment contains the first two dosages (63 mcg and 94 mcg).
Table 2: Dose selection scheme at the beginning of treatment
| Dose | Time* | Dosage (mcg) | Marking of pre-filled syringe/pen |
| Dose 1 | Day 1 | 63 | Orange |
| Dose 2 | Day 14 | 94 | Blue |
| Dose 3 | Day 28 | 125 (full dose) | Grey |
*One injection every 2 weeks (14 days)
A gradual increase in dose at the beginning of treatment contributes to better tolerability of flu-like symptoms that accompany the initiation of interferon use. Prophylactic and concomitant use of anti-inflammatory, analgesic and/or antipyretic drugs may prevent or alleviate the flu-like symptoms that sometimes accompany interferon treatment (see section "Side Effects").
If you accidentally miss a dose of Plegridy
it should be administered as quickly as possible according to the following recommendation:
— If there are 7 days or more until your next scheduled dose:
Give the missed dose immediately. The subsequent dose is administered as planned.
— If there are less than 7 days left until your next scheduled dose:
A new schedule of scheduled injections (once every 2 weeks) should begin on the day of the missed dose. Plegridy should not be administered more frequently than every 7 days.
Use in special patient groups
Elderly patients
In patients over the age of 65 years, the safety and effectiveness of Plegridy have not been sufficiently studied due to the limited number of patients in this age group included in clinical trials.
Kidney failure
According to clinical studies in patients with mild, moderate, severe renal failure, as well as end-stage renal failure, no dose adjustment of Plegridy is required (see sections “Special Instructions” and “Pharmacokinetics”).
Liver failure
Plegridy has not been studied in patients with hepatic impairment (see section "Special Instructions").
Children
The safety and effectiveness of Plegridy have not been studied in patients under 18 years of age, so there are no data on the use of the drug in this age group.
Method of administration
Plegridy is intended for subcutaneous administration.
It is recommended that the patient be taught the correct technique for subcutaneous injections using a prefilled syringe or pen. Patients should be advised to change injection sites. The drug is usually injected under the skin of the abdomen, shoulder or thigh.
Each prefilled syringe or pen comes with a needle. Prefilled syringes and pens are for single use only and should be discarded after use.
Precautions before using the drug
Before injection, the Plegridy drug removed from the refrigerator must be warmed under natural conditions to room temperature (about 30 minutes). The use of external heating sources such as hot water is prohibited.
Within the specified shelf life and in the absence of a refrigerator, the drug can be stored at temperatures up to 25 ° C in a place protected from light for no more than 30 days (see section “Shelf life”).
Prefilled syringes and pens should not be used if the liquid they contain is colored, cloudy, contains visible particles, or has been frozen. The liquid in the syringe or pen should be transparent, colorless or slightly yellow.
PATIENT INSTRUCTIONS FOR USE OF PRE-FILLED SYRINGE AND PEN
SECTION A: PREPARATION FOR INJECTION
Dosage schedule
Pre-filled syringes and pens containing Plegridy are for single use only and should not be reused.
Take the desired prefilled syringe or pen containing Plegridy from the package (Table 3). The pen or prefilled syringe included in the treatment initiation package contains Plegridy for the first two injections, designed to gradually reach the patient's desired dose.
Table 3. Selection of the required packaging of Plegridy
| When | Dose selection | Packaging selection |
| Day 1 (63 mcg) | First injection: 63 mcg, select orange syringe or pen | PACKAGING FOR STARTING A COURSE OF TREATMENT |
| Day 14 (94 mcg) | Second injection: 94 mcg, select blue syringe or pen | |
| Day 28 and every 2 weeks thereafter (125 mcg) | Full dose injection: 125 mcg, select gray syringe or pen | PACKAGING 125 MCG |
It is forbidden
Use more than one prefilled syringe or pen within 14 days (2 weeks).
Syringe pen device
Before use (Figure A)
After application (Figure B)
Pre-filled syringe device
See picture.
Preparing the work surface
Choose a well-lit, clean, level surface, such as a table, and prepare all the materials you will need to perform the injection or self-administer the drug.
Preparation of materials
To perform the injection you will need the following materials:
1. Cotton swab soaked in alcohol
2. Gauze napkin
3. Fixing bandage/adhesive plaster
4. Protective, impermeable container for disposal of used syringes and pens.
Removing a prefilled syringe or pen from the refrigerator
Remove one package of Plegridy from the refrigerator at the dose appropriate for the patient's current treatment program and select the appropriate prefilled syringe or pen from the package as shown in Table 3 (63 mcg, 94 mcg, or 125 mcg).
If there is an unused syringe or pen in the package, close the package and put it back in the refrigerator until the next injection.
Checking the packaging and pre-filled syringe or pen
Check the expiration date printed on the prefilled Plegridy syringe/pen (Figure B), plastic syringe tray, and primary packaging and/or carton.
Do not use
prefilled syringe or pen after the expiration date.
Checking the syringe pen
Check the condition of the syringe pen.
Make sure the green stripes are visible (Figure D).
Do not use the pen if the green bars are not visible in the injection status window.
Make sure that the drug solution in the drug window is not frozen, clear and colorless or slightly yellow (Figure E).
Do not use the Plegridy pen if the solution is frozen, discolored, cloudy, or contains visible particles. It is acceptable if there is an air bubble in the drug solution.
Checking the Prefilled Syringe
Make sure that the drug solution is clear, colorless or slightly yellow.
Do not use the Plegridy syringe if the solution is frozen, discolored, cloudy, or contains visible particles. It is acceptable if there is an air bubble in the drug solution.
Bringing the prefilled syringe/pen to room temperature
Before injecting, leave the prefilled syringe/pen at room temperature for approximately 30 minutes.
Do not use external heat sources such as hot water to warm the prefilled syringe/pen.
Carrying out the injection
The prefilled syringe and pen are intended for subcutaneous administration of Plegridy.
Inject Plegridy using a prefilled syringe or pen and follow the instructions exactly.
Do not inject the drug into an area of the body where the skin is irritated, red, bruised, infected, or scarred. Change injection sites. Do not inject the drug into the same area of the body several times in a row.
Do not remove the cap from the pen or the protective cap from the syringe needle until you are completely ready to inject.
Wash your hands with soap and water.
1. CHOOSE YOUR INJECTION SITE
Select a site to inject the drug and wipe the skin with a swab moistened with alcohol (see picture).
The injection site must be dry before injection. Plegridy should be injected under the skin of the thigh, abdomen or shoulder.
Before administering the drug, do not touch the planned injection site again.
Skip to section B if using a pen, or section C if the injection will be given with a prefilled syringe.
SECTION B: INJECTION USING A PEN
2. REMOVE CAP (Figure E)
Pull the cap and remove it. Do not put the removed cap back on.
The needle is covered with a protective cap, so it is not visible.
Do not touch or press the guard, as you may get stuck by the needle.
The Plegridy syringe pen is ready for injection after removing the cap.
3. POSITIONING THE SYRINGE PEN AND ITS CHECKING (Figure G)
Place the Plegridy pen over the selected injection site. The pen should be held at a 90 degree angle to the injection surface so that the green stripes are visible in the injection status window.
Do not use the pen until you see green bars in the injection status window
4. ADMINISTRATION OF THE DRUG (Figure 3)
Press the syringe pen to the injection site until the clicking stops and green “checkmarks” appear.
Pressure facilitates insertion of the needle and automatic start of the injection. Do not remove the pen from the injection site.
Do not make any movements until the injection is complete.
Continue to press the pen firmly against the skin, still holding it motionless at a 90-degree angle, until the injection is complete.
During the injection (Figure I):
The Plegridy syringe pen will “click” several times.
Green bars will move in the injection status window.
The clicking sounds of the pen will stop when the injection is complete. This should take approximately 5 seconds.
Checking the completion of the injection.
Make sure green checkmarks appear in the injection status window.
5. REMOVING THE SYRINGE PEN FROM THE INJECTION SITE (Figure K)
Lift the Plegridy pen upward from the injection site.
The protective cap will completely cover the needle. Go to Section D, “After Injection,” for instructions on cleaning the injection site and disposing of the Plegridy pen.
SECTION B: INJECTION USING A PRE-FILLED SYRINGE
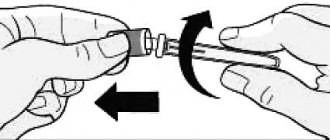
2. SHARPLY REMOVE THE PROTECTIVE CAP FROM THE NEEDLE
Remove the protective cap from the needle and throw it away.
Do not touch the needle.
Do not re-attach the protective cap to the Plegridy syringe.
3. PREPARATION OF THE INJECTION SITE AND POSITION OF THE PRE-FILLED SYRINGE
Gather the treated skin into a fold using your thumb and index finger.
Hold the Plegridy syringe at a 90-degree angle to the injection site.
4. ADMINISTRATION
Quickly insert the needle directly into the fold of skin, all the way to the base of the needle, in a “dart-throwing” fashion.
After inserting the needle, the skin fold can be released.
Slowly press the plunger in one gentle motion until the syringe is completely empty.
This should take about 5 seconds.
Do not remove the needle from the injection site.
5. WAIT 5 SECONDS
The inserted needle should remain under the skin for 5 seconds after completion of the drug administration.
6. REMOVE THE SYRINGE NEEDLE FROM THE INSTRUCTION SITE
Remove the needle while keeping the syringe upright.
Do not re-attach the protective cap to the syringe needle.
Do not reuse the syringe. Go to Section D, After Injection, for instructions on cleaning the injection site and disposing of the syringe.
SECTION D: AFTER INJECTION
Treatment of the injection site
Apply pressure to the injection site for a few seconds using a sterile gauze pad.
If there is blood, blot it.
If necessary, apply a patch.
Syringe pen: checking the completeness of the dose
Check
: green “checkmarks” should be visible in the injection status window (Figure L). Check the medicine window. Make sure that the yellow plunger covers the entire medicine window (Figure M).
A yellow plunger that fills the entire window indicates successful administration of the entire dose of the drug.
The pen syringe should not be reused.
Disposal of syringes and pens
Place the used Plegridy syringe or pen in a special, airtight, protective container, such as a needle disposal container, or a sturdy plastic or metal container with a screw cap, such as detergent or coffee.
Ask your healthcare professional or pharmacy about how to dispose of the container.
There may be local requirements for disposal of needles and syringes.
Do not throw away used syringes or pens containing Plegridy in household waste.
Registration of date and place of injection
Write down the date and location of each injection and rotate injection sites.
Do not inject the drug into the same area of the body several times in a row.
Monitoring the condition of the injection site
After 2 hours, check the injection site for redness, swelling, or tenderness.
If you experience a skin reaction that does not go away within a few days, you should contact your doctor or nurse.
General Warnings
Do not reuse the syringe or pen from Plegridy.
Do not share a syringe or pen containing Plegridy with other patients. Keep the syringe or pen containing Plegridy out of the reach of children.