Description
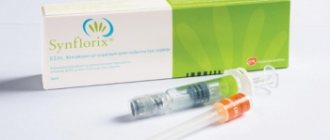
RotaTek is a vaccine for the prevention of rotavirus infection (RVI) in children aged 6 to 32 weeks of life, produced by Merck Charm and Dome, USA.
The vaccine is a 2 ml solution that is administered to the child through the mouth. The vaccination course consists of 3 doses of the vaccine with an interval between doses of 4 to 10 weeks. All 3 vaccinations should be given between 6 and 32 weeks of age, with the first dose of vaccine given between 6 and 12 weeks of age.
Vaccination with the RotaTek vaccine causes the production of specific antibodies to rotavirus serotypes circulating in the Russian Federation. Preventive vaccinations and the Mantoux test after suffering from RVI can be carried out 2-4 weeks after recovery after examination by a doctor.
Before vaccination, a doctor's examination is required on the day of vaccination and for children, the results of a general blood test with leukocyte count and ESR, a general urine test (test results are valid for 4 weeks in the absence of acute diseases during this period). You can also undergo an examination by a doctor at another medical institution; the doctor’s referral requires an indication of the vaccine or a list of diseases for which vaccination is planned, a vaccination certificate or a statement of previous vaccinations.
Rotavirus infection (RVI) is an acute infectious disease affecting primarily the gastrointestinal tract.
RVI is the most common cause of gastroenteritis (inflammatory disease of the stomach and small intestine) reported worldwide. According to WHO, more than 1 billion cases of acute intestinal infections are registered in developing countries per year, including more than 114 million cases of rotavirus gastroenteritis, 20 million of which are severe forms of the disease. Rotavirus infection is associated with 20 to 60% of all hospitalizations among infants with acute intestinal infections.
The causative agent of RVI is a virus of the genus Rotavirus, which includes several antigenic types of human rotavirus: A, B, C, D, E, F, G. The vast majority of cases of RVI in humans are caused by group A rotaviruses.
RVI is an anthroponotic infection, i.e. Transmitted from person to person. Rotaviruses, which cause diseases in animals, are not pathogenic for humans.
Sick children, especially at the onset of the disease and up to 3-5 days from the onset of the disease, excrete rotavirus infection with feces into the external environment. Isolation of the virus is also possible in an apparently healthy child who is experiencing an asymptomatic form of the disease.
A child is infected through contact (through contaminated household items, toys, clothing, bedding, etc.) or through food and water contaminated with the virus.
Rotavirus, released into the external environment, retains its viability for a long time and is relatively resistant to commonly used disinfectants.
The incubation period (the time from the virus entering the body to the development of clinical symptoms) for RVI is quite short and can last from 1 to 3 days.
Contraindications:
- Hypersensitivity to any component of the RotaTek® vaccine, as well as a history of administration of the RotaTek® vaccine.
- History of intussusception.
- Congenital malformations of the gastrointestinal tract predisposing to intussusception.
- Immunodeficiency, suspected immunodeficiency - acute infectious and non-infectious diseases, exacerbation of chronic diseases are temporary contraindications for vaccinations.
- Fructose intolerance, malabsorption of the glucose-galactose complex, and sufficiency of the enzymes sucrase and/or isomaltase.
After the end of the incubation period of the disease, the following symptoms of the disease develop:
- General intoxication: increased body temperature, chills, weakness, headache, dizziness.
- Gastroenteritis: repeated vomiting, abdominal pain, frequent watery foamy stools, flatulence.
- Catarrhal symptoms: redness of the mucous membrane of the oropharynx, sore throat.
The disease can begin either with an increase in body temperature or with repeated vomiting and liquid diarrhea. The child's temperature usually rises to 38-39 degrees Celsius.
If the course of the disease is favorable, its duration is usually 5-7 days. However, in some cases, especially in young children and adults with poor health, various complications may develop.
Who produces Rotatek and what is known about it?
The manufacturer of the drug is the American company Merck&Co, Inc. The drug underwent extensive testing and was approved by the FDA in 2006. The trial data is publicly available: it involved more than 71,000 children, it was a randomized, placebo-controlled trial that strictly complied with all the principles of evidence-based medicine.
Complications
The most common complication of RVI is the development of dehydration (dehydration), especially in children of the first years of life, which in the initial stages is manifested by the following symptoms:
- excitement, anxiety;
- thirst;
- dry skin and mucous membranes;
- slight decrease in skin elasticity;
- slight retraction of the large fontanel.
As dehydration progresses, the child becomes lethargic and lethargic, may refuse to drink, the elasticity of the skin decreases (skin folds straighten out slowly), breathing becomes more frequent, there is a decrease in the frequency of urination and a decrease in the volume of urine excreted. The urine becomes dark and has a pungent odor. In the absence of adequate medical care, life-threatening complications may develop.
Other possible complications of rotavirus infection:
- addition of a bacterial infection;
- acute renal failure,
- damage to the central nervous system in the form of impaired consciousness, seizures and other complications.
Use with other vaccines
The drug RotaTek® can be administered to children simultaneously with any of the following antigens included in both monovalent and combined vaccines: diphtheria toxoid, tetanus toxoid, acellular pertussis vaccine, conjugate vaccine against Haemophilus influenzae type b, inactivated polio vaccine, vaccine against viral hepatitis B , hexavalent vaccine (containing the above components), conjugate pneumococcal vaccine, conjugate meningococcal serogroup C vaccine. There was no decrease in the immune response with simultaneous administration of several vaccines and the RotaTek® vaccine.
| Name of service | Price |
| Vaccine Rota Tech | RUB 3,150 |
Treatment
The basis of treatment for rotavirus infection is adequate fluid replacement (rehydration) and diet.
At home treatment for initial symptoms of dehydration, rehydration is carried out by introducing additional fluid inside. Preference is given not to ordinary water, but to special solutions for rehydration, which are sold in pharmacies (Rehydron, Hydrovit, etc.). Also during the treatment period, dried fruit compote, tea, and 5% glucose solution can be used. You can alternate giving the patient water and the indicated solutions. The liquid should be at room temperature, and should be given to drink in small increments every 5-10 minutes.
If a patient has developed severe dehydration, he needs timely hospitalization for infusion therapy (intravenous administration of special solutions) and other therapy.
Medications for the treatment of RVI should include drugs from the group of enterosorbents, enzyme preparations, and probiotics, which are prescribed by the attending physician.
Antibacterial therapy for uncomplicated, non-severe forms of RVI is not indicated.
Diet is an important component of the treatment of acute intestinal infections
In case of mild RVI, split meals are recommended (5-6 times a day) with small volumes of food prepared by boiling or steaming, mechanically processed (in liquid or pureed form). Whole milk and other dairy products, fresh fruits and vegetables, and legumes are excluded. When the patient’s condition stabilizes and the symptoms of the disease disappear, the diet is gradually expanded. Nutrition correction for infants is carried out by a pediatrician.
Carefully:
- For active diseases of the gastrointestinal tract, including chronic diarrhea (lack of clinical data);
- In case of developmental delay (lack of clinical data).
- In an immunocompromised state (for example, as a result of malignancy or immunosuppressive therapy).
- In close contact with immunocompromised persons (for example, persons with malignant neoplasms or persons receiving immunosuppressive therapy).
- When transfusion of blood or blood products, including immunoglobulins, less than 2 days before the scheduled vaccination.
Specific prevention (vaccine prevention)
Vaccination against rotavirus infection in the Russian Federation is carried out in accordance with the provisions of the Calendar of Preventive Vaccinations for Epidemic Indications (Order of the Ministry of Health of the Russian Federation dated March 21, 2014 No. 125n, Appendix No. 2) and is recommended for active immunization of infants in order to prevent diseases caused by rotaviruses.
The RotaTek vaccine against rotavirus infection is currently registered in Russia. This vaccine is used for specific prevention of RVI in children aged 6 to 32 weeks.
The vaccine is a 2 ml solution that is administered to the child through the mouth. The vaccination course consists of 3 doses of the vaccine with an interval between doses of 4 to 10 weeks. All 3 vaccinations should be given between 6 and 32 weeks of age, with the first dose of vaccine given between 6 and 12 weeks of age.
Vaccination with the RotaTek vaccine causes the production of specific antibodies to rotavirus serotypes circulating in the Russian Federation.
Preventive vaccinations and the Mantoux test after rotavirus infection can be carried out 15-30 days after recovery after examination by a doctor.
Immunobiological properties
Based on pooled REST and FES data, the reduction in hospitalizations and emergency room visits for rotavirus gastroenteritis within 3 years of vaccination was 94.4% (95% CI: 91.6, 96.2) for genotypes G1-G4. 95.5% (95% CI: 92.8; 97.2) for the GJ genotype, 81.9% (95% CI: 16.1; 98.0) for the G2 genotype, 89.0% (95% CI : 53.3; 98.7) for genotype G3, 83.4% (95% CI: 51.2; 95.8) for genotype G4 and 94.2% (95% CI: 62.2; 99.9) for genotype G9.
Clinical studies have confirmed that to achieve the required level and duration of protection against rotavirus gastroenteritis, a full course of vaccination with 3 doses should be administered. However, a retrospective analysis of the data showed that even before completion of the full course of vaccination, the number of cases of rotavirus gastroenteritis with a severity that would require hospitalization or emergency care was reduced (approximately 14 days after the first dose).
What side effects may there be?
Despite the fact that Rotatec is most often well tolerated, side effects are sometimes observed during vaccination. Among them:
- loss of appetite;
- apathy;
- excessive sleepiness;
- rashes on the skin;
- loose stool;
- hyperthermia.
If such symptoms appear, you need to carefully monitor the baby’s condition.
If you notice a deterioration in your health, and side effects become pronounced, you need to seek help from a doctor.
Preparing for vaccination
No special preparation is required for vaccination. At the appointment, the doctor will conduct an examination, assess the baby’s condition and determine the presence or absence of contraindications. Preliminary tests and taking any medications are not required.
Feeding or drinking other liquids immediately before or after vaccination does not affect its effectiveness.
If the child spits out or regurgitates part of the dose, there is no need to re-administer the dose; the administration of the remaining doses continues according to the standard regimen.