Home | About us | Delivery | Advertisers | Login | Registration
The pharmacy is closed on Sundays and holidays.
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2021 Pharmacy 84.
Cocarnit (liof.d/sol. i.m. 187.125 amp. 2ml No. 3+sol.)
A country
Egypt
The country of production may vary depending on the batch of goods. Please check with the operator for detailed information when confirming your order.
Compound
1 ampoule contains: triphosadenine disodium trihydrate 10 mg, cocarboxylase 50 mg, cyanocobalamin 0.5 mg, nicotinamide 20 mg.
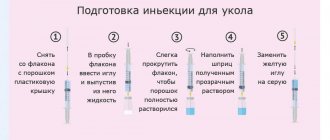
Excipients: glycine - 105.875 mg, methyl parahydroxybenzoate - 0.6 mg, propyl parahydroxybenzoate - 0.15 mg. Solvent: lidocaine hydrochloride - 10 mg, water for injection - up to 2 ml. Lyophilisate for preparing a solution for intramuscular administration in the form of a pink lyophilized mass. The reconstituted solution is transparent and pink in color.
pharmachologic effect
The drug is a rationally selected complex of metabolic substances and vitamins. Trifosadenine is an adenosine derivative that stimulates metabolic processes. Has a vasodilating effect, incl. to the coronary and cerebral arteries. Improves metabolism and energy supply to tissues. Has hypotensive and antiarrhythmic effects. Under the influence of ATP, blood pressure decreases, smooth muscles relax, and the conduction of nerve impulses improves. Cocarboxylase is a coenzyme formed in the body from thiamine (vitamin B1) supplied from outside. It is part of the carboxylase enzyme, which catalyzes the carboxylation and decarboxylation of β-keto acids. Indirectly promotes the synthesis of nucleic acids, proteins and lipids. Reduces the concentration of lactic and pyruvic acids in the body and promotes the absorption of glucose. Improves trophism of nervous tissue. Cyanocobalamin (vitamin B12) is converted into methylcobalamin and 5-deoxyadenosylcobalamin in the body. Methylcobalamin is involved in the conversion of homocysteine to methionine and S-adenosylmethionine - key reactions in the metabolism of pyrimidine and purine bases (hence DNA and RNA). If there is insufficiency of the vitamin in this reaction, it can be replaced by methyltetrahydrofolic acid, and folate-requiring metabolic reactions are disrupted. 5-deoxyadenosylcobalamin serves as a cofactor in the isomerization of L-methylmalonyl-CoA to succinyl-CoA, an important reaction in carbohydrate and lipid metabolism. Vitamin B12 deficiency leads to impaired proliferation of rapidly dividing cells of hematopoietic tissue and epithelium, as well as impaired formation of the myelin sheath of neurons. Nicotinamide is one of the forms of vitamin PP, participates in redox processes in the cell, improves carbohydrate and nitrogen metabolism, and regulates tissue respiration.
Indications for use
Symptomatic treatment of diabetic polyneuropathy.
Mode of application
The drug is injected deep IM (into the gluteal muscle). In cases of severe pain, it is advisable to start treatment with intramuscular administration of 2 ml (1 amp.)/day until acute symptoms subside. Duration of use: 9 days. After improvement of symptoms or in cases of moderately severe symptoms of polyneuropathy, 2 ml (1 amp.) is prescribed 2-3 times a week for 2-3 weeks. The recommended course of treatment is 3-9 injections depending on the severity of the disease. The duration of treatment and repeated courses are determined by the doctor depending on the nature and severity of the disease.
Interaction
In patients using hypoglycemic agents of the biguanide group (metformin), due to impaired absorption of cyanocobalamin from the gastrointestinal tract, a decrease in the concentration of cyanocobalamin in the blood may be observed. Drug interactions with other hypoglycemic agents have not been described. Cyanocobalamin is not compatible with ascorbic acid, heavy metal salts, thiamine, thiamine bromide, pyridoxine, riboflavin, folic acid. Cyanocobalamin should not be used simultaneously with drugs that increase blood clotting. In addition, concomitant use of cyanocobalamin with chloramphenicol should be avoided. Aminoglycosides, salicylates, antiepileptic drugs, colchicine, potassium preparations reduce the absorption of cyanocobalamin. When combined with drugs containing triphosadenine and dipyridamole, the effect of dipyridamole is enhanced, in particular its vasodilating effect. Dimiridamol enhances the effect of triphosadenine. Some antagonism appears when the drug is used together with purine derivatives (caffeine, theophylline). It should not be administered simultaneously with cardiac glycosides in large doses, as the risk of adverse reactions from the cardiovascular system increases. When used simultaneously with xanthinol nicotinate, the effect of the drug is reduced. Nicotinamide potentiates the effect of sedatives, tranquilizers, and antihypertensive drugs.
Side effect
The frequency of adverse reactions is given in accordance with the WHO classification: very often (more than 1/10); often (less than 1/10, but more than 1/100); uncommon (less than 1/100, but more than 1/1000); rare (less than 1/1000, but more than 1/10,000); very rare (less than 1/10,000), including isolated cases; frequency unknown. From the immune system: rarely - allergic reactions (skin rash, difficulty breathing, anaphylactic shock, Quincke's edema). From the nervous system: very rarely - dizziness, headache, agitation, confusion. From the cardiovascular system: - very rarely - tachycardia; - in some cases, bradycardia, arrhythmia; — frequency unknown — pain in the heart area, redness of the skin of the face and upper half of the body with a tingling and burning sensation, “hot flashes.” From the gastrointestinal tract: very rarely - vomiting, diarrhea. From the skin and subcutaneous tissues: very rarely - increased sweating, acne, itching, urticaria. From the side of musculoskeletal and connective tissue: very rarely - convulsions. General disorders and disorders at the injection site: very rarely - irritation, pain and burning at the injection site, weakness may occur. If any of these adverse reactions worsen or any other adverse reactions not listed in the instructions appear, the patient should inform the doctor. If severe adverse reactions develop, the drug is discontinued.
Contraindications
- hypersensitivity to any component of the drug or solvent; - cardiovascular diseases (acute heart failure, acute myocardial infarction, uncontrolled arterial hypertension, arterial hypotension, severe forms of bradyarrhythmias, AV block II-III degree, chronic heart failure (III-IV functional class according to NYHA), cardiogenic shock and others types of shock, QT prolongation syndrome, thromboembolism, hemorrhagic stroke); - inflammatory lung diseases, COPD, bronchial asthma; - pregnancy; - period of breastfeeding; — age up to 18 years; - hypercoagulation (including in acute thrombosis), erythremia, erythrocytosis; - peptic ulcer of the stomach or duodenum in the acute phase; - gout; - hepatitis, cirrhosis of the liver. The drug should be used with caution for angina pectoris.
Overdose
The components of the drug Cocarnit have a wide therapeutic range. Symptoms Trifosadenine. Exceeding the maximum daily dose (about 600 mg for an adult) can lead to the development of the following symptoms: dizziness, decreased blood pressure, short-term loss of consciousness, arrhythmia, AV block of the second and third degrees, asystole, bronchospasm, ventricular disorders, sinus bradycardia and tachycardia. Cocarboxylase. The following symptoms have been reported after administration of more than 100 times the recommended dose: headache, muscle spasm, muscle weakness, paralysis, arrhythmia. Cyanocobalamin. After parenteral administration of a high dose, eczematous skin disorders and benign acne were observed. When used in high doses, hypercoagulation and disruption of purine metabolism may develop. Nicotinamide. When used in large doses, hyperpigmentation, jaundice, amblyopia, weakness, and exacerbation of gastric and duodenal ulcers were observed. With long-term use, the development of steatohepatosis, an increase in the concentration of uric acid in the blood, and impaired glucose tolerance were noted. Treatment Administration of the drug is stopped immediately, symptomatic therapy is prescribed, incl. desensitizing.
special instructions
If the symptoms of the disease worsen or there is no effect after 9 days, it is necessary to correct the course of treatment. When using the drug Cocarnit, proper selection of the dose of the hypoglycemic drug and adequate control of the course of diabetes mellitus are necessary. The color of the prepared solution should be pink. Do not use the drug if the color of the solution has changed. The solution must be used immediately after its preparation. Use in pediatrics There are no data on the effectiveness and safety of the drug Cocarnit in children. Impact on the ability to drive vehicles and operate machinery If side effects from the central nervous system (dizziness, confusion) occur, it is recommended to refrain from driving vehicles and other mechanisms.
Dispensing conditions in pharmacies
On prescription
Side effects
The frequency of adverse reactions is given in accordance with the WHO classification: very often (more than 1/10); often (less than 1/10, but more than 1/100); uncommon (less than 1/100, but more than 1/1000); rare (less than 1/1000, but more than 1/10000); very rare (less than 1/10000), including isolated cases; frequency unknown.
From the immune system: rarely - allergic reactions (skin rash, difficulty breathing, anaphylactic shock, Quincke's edema).
From the nervous system: in some cases - dizziness, headache, agitation, confusion.
From the side of the heart: very rarely - tachycardia; in some cases - bradycardia, arrhythmia; frequency unknown - pain in the heart area.
From the side of blood vessels: frequency unknown - redness of the skin of the face and upper half of the body with a feeling of tingling and burning, hot flashes.
From the gastrointestinal tract: in some cases - vomiting, diarrhea.
From the skin and subcutaneous tissues: very rarely - increased sweating, acne, itching, urticaria.
On the part of the musculoskeletal and connective tissue: very rarely - convulsions.
General disorders and disorders at the injection site: very rarely - irritation, pain and burning at the injection site, weakness.
If any of these adverse reactions worsen or any other adverse reactions not listed in the instructions appear, you must inform your doctor.
If severe adverse reactions develop, the drug is discontinued.
Pharmacokinetics
Trifosadenine. After parenteral administration, it penetrates into organ cells, where it is broken down into adenosine and inorganic phosphate, releasing energy. Subsequently, the breakdown products are included in the resynthesis of ATP.
Cocarboxylase. Rapidly absorbed after intramuscular administration. Penetrates into most tissues of the body. Subject to metabolic decomposition. Metabolic products are excreted primarily by the kidneys.
Cyanocobalamin. In the blood, cyanocobalamin binds to transcobalamins I and II, which transport it to tissues. Deposited mainly in the liver. Communication with plasma proteins - 90%. It is quickly and completely absorbed after intramuscular and subcutaneous administration. Cmax after IM administration is achieved within 1 hour.
It is excreted from the liver by bile into the intestines and again absorbed into the blood. T1/2 - 500 days. Excreted with normal renal function - 7-10% by the kidneys, about 50% by the intestines. With reduced renal function, 0–7% occurs in the kidneys, 70–100% in the intestines.
Penetrates through the placental barrier into breast milk.
Nicotinamide. Quickly distributed into all tissues. Penetrates through the placental barrier and into breast milk. Metabolized in the liver to form nicotinamide-N-methylnicotinamide. Excreted by the kidneys. T1/2 from plasma is about 1.3 hours, steady-state Vd is about 60 l, total clearance is about 0.6 l/min.
Compound
| Lyophilisate for preparing a solution for intramuscular administration | 1 amp. |
| active substances: | |
| triphosadenine disodium trihydrate | 10 mg |
| cocarboxylase | 50 mg |
| cyanocobalamin | 0.5 mg |
| nicotinamide | 20 mg |
| excipients: glycine - 105.875 mg; methyl parahydroxybenzoate - 0.6 mg; propyl parahydroxybenzoate - 0.15 mg | |
| solvent, 1 amp.: lidocaine hydrochloride - 10 mg; water for injection - up to 2 ml |
Contraindications
hypersensitivity to any component of the drug or solvent;
cardiovascular diseases: acute myocardial infarction, uncontrolled arterial hypertension, arterial hypotension, severe forms of bradyarrhythmias, AV block II-III degrees, chronic heart failure (III-IV degrees according to NYHA), cardiogenic shock and other types of shocks, QT prolongation syndrome, thromboembolism, hemorrhagic stroke;
inflammatory lung diseases, chronic obstructive pulmonary diseases, bronchial asthma;
hypercoagulation (including in acute thrombosis), erythremia, erythrocytosis;
peptic ulcer of the stomach or duodenum in the acute stage;
gout;
hepatitis, liver cirrhosis;
pregnancy;
breastfeeding period;
children under 18 years of age.
With caution: angina pectoris.
Overdose
Symptoms:
- triphosadenine (exceeding the maximum daily dose - about 600 mg for an adult) - dizziness, decreased blood pressure, short-term loss of consciousness, arrhythmia, AV block of II and III degrees, asystole, bronchospasm, ventricular disorders, sinus bradycardia and tachycardia;
- cocarboxylase (after administration of a dose exceeding the recommended dose by more than 100 times) - headache, muscle spasm, muscle weakness, paralysis, arrhythmia;
- cyanocobalamin (after parenteral administration of a high dose) - eczematous skin disorders and benign acne. Possible development of hypercoagulation, disruption of purine metabolism;
- nicotinamide (when using large doses) - hyperpigmentation, jaundice, amblyopia, weakness, exacerbation of gastric and duodenal ulcers. With long-term use, the development of steatohepatosis, an increase in the concentration of uric acid in the blood, and impaired glucose tolerance were noted.
Treatment: immediate cessation of drug administration, symptomatic therapy, incl. desensitizing.
The components of the drug Cocarnit have a wide therapeutic range.
Release form
Lyophilisate for preparing a solution for intramuscular administration/complete with solvent. 187.125 mg of the drug in dark glass ampoules with one or two broken rings, volume 3 ml.
2 ml of solvent (0.5% lidocaine hydrochloride solution) in 2 ml dark glass ampoules with a break ring.
3 amp. with the drug complete with 3 amp. solvent in a blister pack.
1 blister pack is placed in a cardboard pack.
Interaction
In patients using hypoglycemic agents of the biguanide group (metformin), due to impaired absorption of cyanocobalamin from the gastrointestinal tract, a decrease in the concentration of cyanocobalamin in the blood may be observed. No drug interactions have been described with other hypoglycemic agents.
Cyanocobalamin is incompatible with ascorbic acid, heavy metal salts, thiamine bromide, pyridoxine, riboflavin, and folic acid. Cyanocobalamin should not be used simultaneously with drugs that increase blood clotting. In addition, concomitant use of cyanocobalamin with chloramphenicol should be avoided. Aminoglycosides, salicylates, antiepileptic drugs, colchicine, potassium preparations reduce the absorption of cyanocobalamin.
When drugs containing triphosadenine are used together with dipyridamole, the effect of dipyridamole, in particular the vasodilating effect, is enhanced. Dipyridamole enhances the effect of triphosadenine.
Some antagonism appears when the drug is used together with purine derivatives (caffeine, theophylline).
It should not be administered simultaneously with cardiac glycosides in large doses, since the risk of developing adverse reactions from the cardiovascular system increases.
When used simultaneously with xanthinol nicotinate, the effect of the drug is reduced.
Nicotinamide potentiates the effect of sedatives, tranquilizers, and antihypertensive drugs.
Pharmacodynamics
The drug is a rationally selected complex of metabolic substances and vitamins.
Trifosadenine is an adenosine derivative that stimulates metabolic processes. Has a vasodilating effect, incl. to the coronary and cerebral arteries. Improves metabolism and energy supply to tissues. Has hypotensive and antiarrhythmic effects. After parenteral administration, it penetrates into organ cells, where it is broken down into adenosine and inorganic phosphate, releasing energy. Subsequently, the breakdown products are included in ATP synthesis. Under the influence of ATP, blood pressure decreases, smooth muscles relax, and the conduction of nerve impulses improves.
Cocarboxylase is a coenzyme formed in the body from thiamine (vitamin B1) supplied from outside. Part of the enzyme carboxylase, which catalyzes the carboxylation and decarboxylation of α-keto acids. Indirectly promotes the synthesis of nucleic acids, proteins and lipids. Reduces the concentration of lactic and pyruvic acids in the body and promotes the absorption of glucose. Improves trophism of nervous tissue.
Cyanocobalamin (vitamin B12) is converted into methylcobalamin and 5-deoxyadenosylcobalamin in the body. Methylcobalamin is involved in the conversion of homocysteine to methionine and S-adenosylmethionine - key reactions in the metabolism of pyrimidine and purine bases (and therefore DNA and RNA). If there is insufficiency of the vitamin in this reaction, it can be replaced by methyltetrahydrofolic acid, which disrupts folate-dependent metabolic reactions.
5-deoxyadenosylcobalamin serves as a cofactor in the isomerization of L-methylmalonyl-CoA to succinyl-CoA, an important reaction in carbohydrate and lipid metabolism.
Vitamin B12 deficiency leads to impaired proliferation of rapidly dividing cells of hematopoietic tissue and epithelium, as well as impaired formation of the myelin sheath of neurons.
Nicotinamide is one of the forms of vitamin PP, participates in redox processes in the cell, improves carbohydrate and nitrogen metabolism, and regulates tissue respiration.