Oxyten tablets p/o 20 mg No. 10x1
Name
Oxyten.
Description
Beige, film-coated tablets.
Main active ingredient
Tenoxicam.
Release form
Pills.
Dosage
Pharmacological group
Nonsteroidal anti-inflammatory and antirheumatic drugs. Oxycams.
Pharmacodynamics
The drug Oxyten is a non-steroidal anti-inflammatory drug (NSAID), which has a pronounced and long-lasting analgesic, anti-inflammatory, and antipyretic effect. As with other NSAIDs, the exact mechanism of action is not known; it may be multifactorial and includes inhibition of prostaglandin synthesis and reduces the accumulation of leukocytes at the site of inflammation.
Pharmacokinetics
Oxyten is a long-acting drug; a single daily dose is effective. The drug is quickly and completely absorbed in unchanged form after oral administration. Taking along with food reduces the rate, but does not affect the amount of absorption of the drug Oxyten. Tenoxicam penetrates well into the synovial fluid, reaching concentrations approximately half those in plasma. The average plasma half-life is approximately 72 hours. When using the recommended dose of 20 mg once daily, the equilibrium plasma concentration is maintained for 10-15 days, with no unexpected accumulation observed. Tenoxicam binds tightly to plasma proteins. Tenoxicam is eliminated from the body primarily through metabolic pathways. About 2/3 of the administered dose is excreted in the urine (in the form of the inactive 5-hydroxy pyridyl metabolite), and the rest in the bile (in the form of the glucuronic conjugate hydroxymetabolite). No changes in the pharmacokinetics of the drug Oxyten depending on the age of patients were observed, despite individual changes in trends towards an increase in such characteristics in older patients.
Indications for use
Oxythene is indicated for the relief of pain and inflammation in osteoarthritis and rheumatoid arthritis. Oxyten is also used for short-term treatment of acute diseases of the musculoskeletal system, including sprains, dislocations and other soft tissue injuries.
Directions for use and doses
Adults: The recommended dose is 20 mg per day. It is advisable to take it at the same time every day during or after meals with water. Higher doses should not be used, as this does not always achieve a significantly more pronounced therapeutic effect, and the risk of adverse events increases. Duration of therapy: Treatment of acute musculoskeletal disorders usually does not exceed 7 days. In exceptional cases, the duration of therapy can be extended to 14 days. Elderly patients: Prescribe with caution, because against the background of concomitant treatment or impaired renal, hepatic or cardiovascular function, the likelihood of side effects increases compared to younger patients. If tenoxicam is necessary, the lowest effective dose and the shortest possible treatment period should be prescribed. During treatment with NSAIDs, patients should be regularly monitored for gastrointestinal bleeding during the first 4 weeks. Children: There is not enough information on the use of tenoxicam in children. At sufficiently low concentrations of albumin in the blood plasma (eg, nephrotic syndrome) or at high concentrations of bilirubin, use with caution due to the high affinity of tenoxicam for plasma proteins. In case of renal failure, patients with creatinine clearance more than 25 ml/min require careful medical supervision without adjusting the dosage regimen. In patients with creatinine clearance less than 25 ml/min, use with caution due to the lack of sufficient information on the use of tenoxicam in such patients. Use with caution in patients with impaired liver function due to the lack of sufficient information on the use of tenoxicam in such patients.
Use during pregnancy and lactation
Pregnancy: Inhibition of prostaglandin synthesis may affect pregnancy and/or fetal development. Evidence from epidemiological studies suggests an increased risk. Congenital anomalies caused by NSAID use have been reported, but the incidence is low and no consistent pattern has been found. Taking into account the known effects of NSAIDs on the fetal cardiovascular system (risk of closure of the ductus arteriosus), the use of NSAIDs in the third trimester of pregnancy is contraindicated. The onset of labor may be delayed, and its duration may increase; There is also a tendency for increased bleeding in mother and child. The use of NSAIDs in the first two trimesters of pregnancy and during childbirth is possible only in cases where the potential benefit to the patient outweighs the potential risk to the fetus. In the third trimester of pregnancy, all prostaglandin synthesis inhibitors can expose the fetus to: cardiopulmonary toxicity (with premature closure of the ductus arteriosus and pulmonary hypertension); renal dysfunction, which may progress to renal failure with oligohydramnios. At the same time, the mother at the end of pregnancy and the newborn may experience: an increase in bleeding time, antiplatelet effects that can occur even from low doses; inhibition of uterine contractions, which may delay or prolong labor. Therefore, tenoxicam is contraindicated in the third trimester of pregnancy. Lactation: A few studies have shown that NSAIDs pass into breast milk in very low concentrations. If possible, the use of NSAIDs should be avoided during breastfeeding.
Precautionary measures
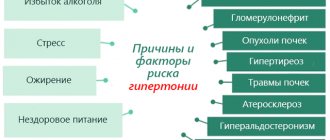
Oxyten should not be used with other NSAIDs, including selective cyclooxygenase-2 inhibitors. Undesirable effects can be minimized by using the lowest effective dose for the shortest period of time. Cardiovascular and cerebrovascular effects Appropriate monitoring and counseling of patients with hypertension and/or mild to moderate congestive heart failure is recommended, as fluid retention and edema have been reported with the use of NSAIDs. Clinical trial and epidemiological data indicate that the use of some NSAIDs (particularly at high doses and long-term treatment) may be associated with a small increased risk of arterial thrombotic events (eg, myocardial infarction or stroke). Available data are insufficient to exclude a similar risk with tenoxicam. Therefore, long-term treatment with tenoxicam in patients with risk factors for cardiovascular disease, as well as patients with uncontrolled hypertension, congestive heart failure, established coronary artery disease, peripheral arterial vascular disease and/or cerebrovascular disease, is possible only after careful consideration of the case. Similar recommendations should be taken into account before initiating long-term treatment in patients with risk factors for cardiovascular disease (such as hypertension, hyperlipidemia, diabetes mellitus, smoking). Cardiovascular, renal and hepatic failure The use of NSAIDs can cause a dose-dependent decrease in prostaglandin formation and the occurrence of induced renal failure. Patients taking diuretics and the elderly are at greater risk of this reaction. Such patients should be monitored for renal function. Isolated cases of increased levels of serum transaminases or other indicators of liver function have been reported. In most cases, data above the normal range of values were weak and transient. If there is a significant or persistent deviation, you should stop using the drug Oxyten and repeat the tests. Particular care must be taken when treating patients with existing liver disease. In rare cases, NSAIDs can cause interstitial nephritis, glomerulonephritis, papillary necrosis and nephrotic syndrome. Such substances inhibit renal prostaglandin synthesis, which plays an auxiliary role in maintaining renal perfusion in patients with reduced blood flow and blood volume. The use of NSAIDs in these patients may cause clinical renal decompensation with a return to the pre-therapy state after cessation of treatment. Patients at greatest risk for this reaction are those with existing renal disease (including patients with diabetes and renal impairment), nephritic syndrome, increased interstitial fluid volume, liver disease, heart failure, and patients receiving concurrent treatment with diuretics or potentially nephrotoxic agents. In such patients, careful monitoring of renal, liver and cardiac function should be established. The dosage used should be minimal. NSAIDs should be used with caution in patients with a history of heart failure or hypertension. Dermatological effects Serious skin reactions, including exfoliative dermatitis, Stevens-Johnson syndrome and toxic epidermal necrolysis, have been very rarely reported with the use of NSAIDs. The risk of developing such reactions is highest at the beginning of treatment: the first manifestation was noted during the first month of therapy. At the first signs of skin rash, lesions of the mucous membranes or other signs of hypersensitivity, you should stop using the drug. Elderly patients Elderly patients have an increased incidence of adverse reactions to NSAIDs, especially gastrointestinal bleeding and perforation. Particular care should be taken and regular monitoring of elderly patients should be carried out to detect possible interactions with concomitant drugs and monitoring of renal, hepatic and cardiovascular functions that may be affected by NSAIDs. Impaired fertility in women The use of the drug may impair the fertility of women, therefore its use is not recommended for women planning pregnancy. Gastrointestinal bleeding, ulceration and perforation Caution should be exercised when using NSAIDs in patients with a history of gastrointestinal disease. Gastrointestinal bleeding, ulceration and perforation have been reported with all NSAIDs at any time during treatment, with or without warning symptoms or previous serious gastrointestinal events. The risk of gastrointestinal bleeding, ulceration, or perforation increases with increasing doses of NSAIDs in patients with a history of ulcers, especially those complicated by bleeding or perforation, and in elderly patients. Treatment of such patients should begin with the lowest possible dose. For such patients, treatment in combination with protective agents (eg, misoprostol or proton pump inhibitors) should be considered, as well as for patients taking concomitant low-dose aspirin or other drugs that may increase the risk of gastrointestinal damage. Patients with a history of GI toxicity, especially older patients, should report unusual abdominal symptoms (especially GI bleeding), especially during the initial stages of treatment. Caution should be exercised when treating patients concomitantly taking medications that may increase the risk of ulceration or bleeding, such as oral corticosteroids, anticoagulants (such as warfarin), selective serotonin reuptake inhibitors, or antiplatelet agents (such as aspirin). Patients taking tenoxicam who have gastrointestinal symptoms should be closely monitored. If a peptic ulcer or gastrointestinal bleeding occurs, you should immediately stop using the drug. NSAIDs should be used with caution in patients with a history of gastrointestinal diseases (ulcerative colitis, Crohn's disease), as exacerbation of these diseases is possible. Hematological effect Tenoxicam reduces platelet aggregation and may increase bleeding time. This should be taken into account when treating patients undergoing major surgery (eg, joint replacement) and when determining bleeding time. Ophthalmic effect Adverse eye effects have been reported with the use of NSAIDs. For this reason, patients who experience visual disturbances during treatment with tenoxicam should undergo an ophthalmological examination. Respiratory disorders Caution should be exercised when treating patients with or with a history of asthma, as NSAIDs have been reported to cause bronchospasm in such patients. Systemic lupus erythematosus and mixed connective tissue disease: Patients with systemic lupus erythematosus and mixed connective tissue disease may have an increased risk of developing aseptic meningitis.
Interaction with other drugs
Other analgesics, including selective cyclooxygenase-2 inhibitors: Concomitant use of two or more NSAIDs (including aspirin) should be avoided as this may increase the risk of unwanted side effects. Acetylsalicylic acid and salicylates: Salicylates can replace protein-bound tenoxicam, thereby increasing the clearance and volume of distribution of tenoxicam. The simultaneous use of salicylates with Oxyten tablets should be avoided, as this may increase the risk of unwanted side effects (mainly gastrointestinal). Antacids and H2-receptor antagonists: Antacids may reduce the rate, but not the amount, of absorption of tenoxicam. These differences are not clinically significant. No interactions were observed with concomitant use of cimetidine. Anticoagulants: Tenoxicam is highly bound to serum albumin and, like all NSAIDs, may enhance the anticoagulant effect of warfarin and other anticoagulants. It is recommended to carefully monitor the effects of anticoagulants and oral glycemic agents, especially during the initial stages of treatment with Oxyten. No interaction with digoxin was observed. In healthy subjects, no clinically significant interaction was observed between tenoxicam and low molecular weight heparin. Antiplatelet agents and selective serotonin reuptake inhibitors (SSRIs): When used simultaneously with NSAIDs, the risk of gastrointestinal bleeding increases. Antihypertensives: Tenoxicam and other NSAIDs may reduce the effect of antihypertensives. Cardiac glycosides: NSAIDs can exacerbate heart failure, reduce the glomerular filtration rate and increase the level of cardiac glycosides in the blood plasma when used in parallel. Cyclosporine: As with other NSAIDs, caution should be exercised when co-administering cyclosporine as the risk of nephrotoxicity increases. Corticosteroids: As with other NSAIDs, caution should be exercised when taking corticosteroids concomitantly as there is an increased risk of ulcers or gastrointestinal bleeding. Diuretics: The effect of diuretics is reduced. NSAIDs may cause sodium, potassium, and fluid retention and may interfere with the natriuretic effects of diuretics, which may increase the risk of NSAID nephrotoxicity. These features should be taken into account when treating patients with cardiac dysfunction or hypertension, since these effects may cause a deterioration in the patient's condition. Lithium: NSAIDs have been reported to decrease the excretion of lithium. If tenoxicam is present in a patient taking lithium, lithium levels should be monitored frequently and the patient should be alerted to adequate fluid intake and symptoms of lithium toxicity. Methotrexate: Caution should be exercised when taking methotrexate concomitantly, as this increases the risk of increased toxicity since NSAIDs impair the elimination of methotrexate. Mifepristone: NSAIDs should not be used for 8 to 12 days after taking mifepristone, as NSAIDs may reduce the effectiveness of the latter. NSAIDs, selective cyclooxygenase-2 inhibitors, salicylates: Avoid coadministration of two or more NSAIDs (including aspirin) as this may increase the risk of adverse effects. Salicylates are able to displace tenoxicam from protein binding sites and thus increase the clearance and volume of distribution of the drug Oxyten. Concomitant treatment with salicylates or other NSAIDs should be avoided due to the increased risk of adverse reactions (particularly gastrointestinal). Penicillamine and parenteral gold: In a small number of patients receiving parenteral penicillamine or parenteral gold, no clinically significant interaction was observed. Quinolones: Data from animal studies suggest that NSAIDs may increase the risk of seizures caused by quinolone antibiotics. Patients receiving NSAIDs and quinolones may be at increased risk of seizures. Tacrolimus: When NSAIDs are used concomitantly with tacrolimus, there may be an increased risk of nephrotoxicity. Zidovudine: When NSAIDs are used concomitantly with zidovudine, there is an increased risk of hematological toxicity. There is evidence of an increased risk of hemarthrosis and hematoma in HIV-positive patients with hemophilia taking both zidovudine and ibuprofen. Influence on the ability to drive a car and other mechanisms. Patients experiencing adverse effects such as vertigo, dizziness, drowsiness, fatigue, or visual disturbances should avoid driving or operating machinery.
Contraindications
Hypersensitivity, erosive and ulcerative lesions of the gastrointestinal tract (including a history), gastrointestinal bleeding (including a history), severe gastritis; complete or incomplete combination of bronchial asthma, recurrent nasal polyposis and paranasal sinuses and intolerance to acetylsalicylic acid or other non-steroidal anti-inflammatory drugs (NSAIDs) (including a history); hemophilia, hypocoagulation, liver and/or renal failure, inflammatory diseases of the gastrointestinal tract, progressive kidney disease, active liver disease, condition after coronary artery bypass grafting; confirmed hyperkalemia, hearing loss, pathology of the vestibular apparatus, glucose-6-phosphate dehydrogenase deficiency; blood diseases, pregnancy, lactation.
Compound
Each film-coated tablet contains 20 mg tenoxicam; Excipients: lactose monohydrate, corn starch, talc, magnesium stearate, hypromellose, titanium dioxide (E171), yellow iron oxide (E172).
Overdose
There have been no reported cases of serious overdose of Oxyten tablets. There are no specific recommendations for overdose, but the use of H2-histamine receptor blockers may be advisable. Gastric lavage should be performed as soon as possible after ingestion of the drug at an increased dose, while the patient should be carefully monitored and general supportive measures should be implemented if necessary.
Side effect
In most patients, side effects are short-lived and go away without stopping treatment. The most commonly reported adverse events are from the gastrointestinal tract. Cardiovascular and cerebrovascular effects: Edema, hypertension and heart failure caused by the use of NSAIDs have been reported. Rarely, palpitations and shortness of breath have been observed. Clinical trial and epidemiological data indicate that the use of some NSAIDs (particularly at high dosages and long-term treatment) may lead to an increased risk of arterial thrombotic events. Dermatological effects: With the use of some NSAIDs, there have been reports of photosensitivity and bullous reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis (very rare). Gastrointestinal effects: The most common side effects may be observed in the gastrointestinal tract when taking NSAIDs. They include dyspepsia, nausea, vomiting, abdominal pain and discomfort, constipation, diarrhea, flatulence, indigestion, epigastric discomfort, melena, hematemesis, ulcerative stomatitis, anorexia, exacerbation of colitis and Crohn's disease. As with other NSAIDs, there is a risk of peptic ulcers, perforation and gastrointestinal bleeding, especially in elderly patients. Gastritis was less common. The development of pancreatitis has been very rarely reported. Hematological effects: When using NSAIDs, a decrease in hemoglobin is possible, not associated with gastrointestinal bleeding. Anemia, aplastic and hemolytic anemia, thrombocytopenia and non-thrombocytopenic purpura, leukopenia, neutropenia and eosinophilia have been reported. Nosebleeds have been reported infrequently. In rare cases, agranulocytosis has been observed. Liver disorders: Liver dysfunction. As with other NSAIDs, there may be changes in various parameters of liver function. In some patients, serum transaminase levels may increase during treatment. Although the incidence of these reactions is low, if liver function test results are significantly or persistently abnormal, signs and symptoms of liver disease develop, or systemic manifestations (eg, eosinophilia, rash) occur, discontinue use of the drug. Hepatitis and jaundice have been reported. Hypersensitivity: The following hypersensitivity reactions have been reported during treatment with NSAIDs: a) Nonspecific allergic reactions and anaphylaxis; b) Increased airway reactivity, including asthma, exacerbation of asthma, bronchospasm or shortness of breath; c) Various skin disorders, including various types of rashes. Angioedema, itching and purpura have been reported. Nail disorders, alopecia, erythema, urticaria and photosensitivity reactions have been rarely reported. As with other NSAIDs, exfoliative and bullous dermatoses have occurred, including epidermal necrolysis, erythema multiforme and Stevens-Johnson syndrome. Vesiculobullous reactions and vasculitis have been rarely reported. Metabolic disorders: Rarely, metabolic disorders such as weight loss or weight gain and hyperglycemia have been reported. Neurological and sensory disturbances: Visual disturbances, optic neuritis, ocular swelling, blurred vision and eye irritation have been reported. Ophthalmoscopy and slit-lamp examination revealed no changes in the eyes. General malaise and tinnitus may occur. Other less common effects have been reported, including paraesthesia and aseptic meningitis (especially in patients with existing autoimmune diseases such as systemic lupus erythematosus and mixed connective tissue disease) with symptoms such as stiff neck, headache, nausea, vomiting, fever or loss of orientation. Dizziness, malaise, fatigue and hypersomnia. Rarely, headache, drowsiness, insomnia, depression, nervousness, sleep disturbances, clouding of consciousness, paresthesia and vertigo have been reported. Renal disorders: Nephrotoxicity has been reported in various forms, including interstitial nephritis, nephrotic syndrome and renal failure. Reversible increases in blood urea nitrogen and blood creatinine levels were observed. If side effects occur, tell your doctor. This applies to all possible side effects, including those not described in these instructions.
Storage conditions
Store at a temperature of 15-25°C, protected from light. Keep out of the reach of children.
Buy Oxyten tablet p/o 20 mg in blister pack. in pack No. 10x1 in the pharmacy
Price for Oxyten tablet p/o 20 mg per blister. in pack №10x1
Instructions for use for Oxyten tablet p/o 20 mg per blister. in pack №10x1
Brief description of the drug
The drug Oxyten is used to relieve pain and inflammation. The drug has an analgesic effect and relieves fever. The mechanism of action of the drug is based on the fact that the enzyme cyclooxygenase is inhibited, as a result of which the metabolic processes of arachidonic acid begin to be disrupted, and the synthesis of prostaglandins is blocked during the development of inflammation.
Tradename
Oxyten
Active substance
Tenoxicam
Pharmacological group
NSAIDs - non-steroidal anti-inflammatory drugs
Release form and packaging
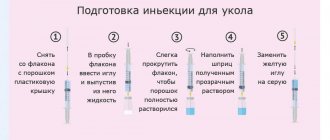
The drug is produced in two forms - tablets and lyophilized powder , which is suitable for preparing injections .
- Beige-coated tablets are available in blisters. One blister contains 10 tablets. The blister is stored in a cardboard box, the packaging includes instructions for use.
- Lyophilized powder for the preparation of solution for injection, yellow or yellow-green, compacted. Supplied complete with a solvent - a transparent, colorless, odorless liquid. The powder is sold in 2 ml bottles. The kit includes instructions for use, 1 ampoule of the drug and 1 ampoule of solvent.
Packaging of Oxyten tablets
Compound
tenoxicam as an active substance in a dosage of 20 mg.
As additional components, Oxyten tablets
- milk sugar;
- starch;
- talc;
- E 572;
- titanium white;
- iron oxide yellow pigment.
The lyophilisate contains as additional components:
- mannitol;
- sodium hydroxide;
- trometamol;
- sodium pyrosulfate;
- trilon B.
Water for injection is included as a solvent in the lyophilisate kit.
Side effects
If the dosage is incorrect due to violation of the rules for taking pills and the injection schedule, the following side effects may develop:
- digestive disorders: NSAID gastropathy, dyspepsia (nausea, diarrhea, vomiting, heartburn, flatulence), abdominal pain, liver dysfunction, stomatitis, anorexia, ulceration of the gastrointestinal mucosa, perforation of the intestinal walls, increased activity of AST, ALT, GGT, bilirubin levels in serum;
- cardiovascular disorders: heart failure, increased blood pressure, tachycardia;
- neurological disorders: dizziness, drowsiness, headache, depression, hearing loss, agitation, tinnitus, blurred vision, eye irritation;
- dermatological reactions: itching, photosensitivity, rashes, severe skin reactions (SCARs), exfoliative dermatitis, erythema, bullous rashes, allergic type purpura;
- urinary disorders: increased levels of urea nitrogen, creatinine in the blood;
- hematological disorders: agranulocytosis, leukopenia, anemia, leukopenia, thrombocytopenia, pancytopenia;
- changes in laboratory parameters: hypercreatininemia, increased concentration of urea nitrogen, activity of liver transaminases, hyperbilirubinemia, prolongation of bleeding time;
- others: mental disorders, metabolic disorders, bleeding (gastrointestinal, hemorrhoidal, uterine).
Before prescribing Oxyten, the patient must undergo a full examination, take tests, and consult with a doctor regarding existing contraindications.
Sometimes it is necessary to take tests to determine allergic reactions and hypersensitivity to the active substance or auxiliary components of the drug.
Tachycardia can be a side effect of taking Oxyten
Reviews from patients and doctors
Oxyten quickly relieves pain and inflammation in joint diseases. But the medication has many contraindications and side effects, so you should not take it yourself. Only a specialist can select an adequate treatment regimen.
Eduard Vladimirovich, rheumatologist
The interphalangeal joints of my hands began to hurt. She could hardly hold the spoon in her hands. I went to the doctor who prescribed Oxyten for me. It is convenient to take, just once a day. The pain began to subside, but I was unable to finish the entire course due to unwanted reactions from the heart.
Lyudmila, 38
I have been suffering from rheumatoid arthritis for a long time. The last time the doctor prescribed Oxyten. The medication relieves pain well, but, unfortunately, does not cure pathology.
Pavel, 37
Pharmacodynamics
Oxyten has a pronounced and long-lasting analgesic, anti-inflammatory, and antipyretic effect.
The mechanism is associated with inhibition of the enzyme cyclooxinase (inhibits the activity of cyclooxygenase 1 and cyclooxygenase 2, which leads to disruption of the metabolism of arachidonic acid and blockade of prostaglandin synthesis in the site of inflammation, as well as other tissues of the body.
In addition, Oxyten reduces the accumulation of leukocytes at the site of inflammation.
For rheumatic diseases, it relieves joint pain at rest and during movement, reduces morning stiffness and swelling.
Oxyten is very effective in the fight against leukocytes, which can be localized in large numbers at the site of inflammation. If a person experiences pronounced intense pain in the joints even at rest, then Oxyten should be taken. The medicine eliminates morning stiffness, swelling and pain when moving.
The drug slows down the glomerular filtration rate, increasing the level of cardiac glycosides in the body.
Oxyten helps with joint pain
Indications for use
The creators of the drug conceived it as a means to combat degenerative changes that occur in various tissues under the influence of inflammatory processes. Therefore, the list of ailments for which the drug is prescribed is very extensive. First of all, these are diseases associated with the musculoskeletal system and musculoskeletal tissues. These include:
- arthritis;
- arthrosis;
- lumbago;
- tissue damage;
- various types of injuries;
- musculoskeletal diseases in the acute stage;
- gout;
- inflammatory and degenerative pathologies of the musculoskeletal system (rheumatoid arthritis, osteoarthritis, arthritis, ankylosing spondylitis);
- tendonitis;
- bursitis;
- radiculitis;
- periarthritis;
- primary dysmenorrhea.
The main feature of the medicine is that it prevents the development of inflammatory processes in the damaged area and relieves pain well. Used for complex therapy for these diseases and acute pain.
The drug reduces pain and relieves inflammation immediately at the time of use. The development of the disease does not stop. Therefore, it is used in complex therapy.
Bursitis is one of the indications for the use of Oxyten