Valocordin-Doxylamine, 1 piece, 20 ml, 25 mg/ml, drops for oral administration
The frequency of potential adverse reactions is classified as follows: very common (≥1/10); often (≥1/100 <1/10); uncommon (≥1/1000 to <1/100); rare (≥1/10000 to <1/1000); very rare (<1/10000) and frequency unknown (cannot be estimated from available data).
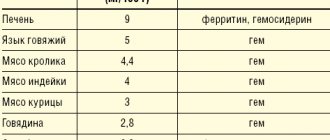
From the blood and lymphatic system:
in exceptional cases, the development of leukopenia, thrombocytopenia, and hemolytic anemia was reported during treatment with H1-histamine receptor blockers; very rarely - cases of aplastic anemia and agranulocytosis have been reported.
From the endocrine system:
in patients with pheochromocytoma, the use of histamine H1 receptor blockers may cause an increase in the release of catecholamines.
Mental disorders:
Adverse reactions, depending on individual susceptibility and the dose taken, include a decrease in the speed of psychomotor reactions, decreased concentration, depression; In addition, paradoxical reactions may occur - restlessness, agitation, anxiety, insomnia, nightmares, confusion, hallucinations, tremor. After prolonged daily use, abrupt cessation of therapy can lead to rebound insomnia.
Drug addiction. Like other sleeping pills, doxylamine can cause physical and mental dependence. The risk of addiction increases with increasing dose and duration of treatment, as well as in patients with alcohol or drug addiction (including a history).
Rebound insomnia. Even after short courses of doxylamine, its withdrawal can lead to a relapse of insomnia. Therefore, treatment should be stopped by gradually reducing the dose.
Anterograde amnesia. Even in therapeutic doses, doxylamine can cause anterograde amnesia, especially within the first hour after its administration. The risk increases with increasing dose, but can be reduced by getting sufficient uninterrupted sleep (7–8 hours).
From the nervous system:
dizziness, drowsiness, headache; rarely - convulsions are possible.
From the side of the organ of vision:
accommodation disturbances, increased IOP.
From the organ of hearing and balance:
noise in ears.
From the SSS side:
tachycardia, arrhythmia, decreased or increased blood pressure, decompensated heart failure. In several cases, ECG changes were detected.
From the respiratory system, chest and mediastinum:
thickening of bronchial secretions, bronchial obstruction, bronchospasm, which can lead to impaired pulmonary function.
From the gastrointestinal tract:
autonomic adverse reactions such as dry mouth and constipation; Nausea, vomiting, diarrhea, decreased or increased appetite, and epigastric pain may occur; very rarely - life-threatening paralytic intestinal obstruction.
From the liver and biliary tract:
Liver dysfunction (cholestatic jaundice) has been reported during antihistamine therapy.
From the skin and subcutaneous tissue:
allergic skin reactions, photosensitivity, impaired thermoregulation.
From the musculoskeletal system and connective tissue:
muscle weakness.
From the kidneys and urinary tract:
urinary disturbance.
General and administration site disorders:
nasal congestion, fatigue, lethargy.
Careful individual selection of the daily dose can reduce the frequency and severity of adverse reactions. The risk of adverse reactions is higher in older patients, which may increase their risk of falls.
Development of addiction:
Regular use may lead to decreased effectiveness (addiction).
VALOCORDIN-DOXYLAMINE 0.025/ML 20ML FL/CAP
The frequency of potential adverse reactions is classified as follows: very common (≥1/10); often (≥1/100 to <1/10); uncommon (≥1/1000 to <1/100); rare (≥1/10000 to <1/1000); very rare (up to <1/10000) and frequency unknown (cannot be estimated from available data).
From the blood and lymphatic system:
in exceptional cases, the development of leukopenia, thrombocytopenia, and hemolytic anemia was reported during treatment with H1-histamine receptor blockers; very rarely - cases of aplastic anemia and agranulocytosis have been reported.
From the endocrine system:
in patients with pheochromocytoma, the use of histamine H1 receptor blockers may cause an increase in the release of catecholamines.
Mental disorders:
Adverse reactions, depending on individual susceptibility and the dose taken, include a decrease in the speed of psychomotor reactions, decreased concentration, depression; In addition, paradoxical reactions may occur - restlessness, agitation, anxiety, insomnia, nightmares, confusion, hallucinations, tremor. After prolonged daily use, abrupt discontinuation of therapy can lead to “rebound” insomnia.
Drug addiction.
Like other sleeping pills, doxylamine can cause physical and mental dependence. The risk of addiction increases with increasing dose and duration of treatment, as well as in patients with alcohol or drug addiction (including a history).
"Rebound" insomnia.
Even after short courses of doxylamine, its withdrawal can lead to a relapse of insomnia. Therefore, treatment should be stopped by gradually reducing the dose.
Anterograde amnesia.
Even in therapeutic doses, doxylamine can cause anterograde amnesia, especially within the first hour after its administration. The risk increases with increasing dose, but can be reduced by getting sufficient uninterrupted sleep (7–8 hours).
From the nervous system:
dizziness, drowsiness, headache; rarely - convulsions are possible.
From the side of the organ of vision:
accommodation disturbances, increased IOP.
From the organ of hearing and balance:
noise in ears.
From the SSS side:
tachycardia, arrhythmia, decreased or increased blood pressure, decompensated heart failure. In several cases, ECG changes were detected.
From the respiratory system, chest and mediastinum:
thickening of bronchial secretions, bronchial obstruction, bronchospasm, which can lead to impaired pulmonary function.
From the gastrointestinal tract:
autonomic adverse reactions such as dry mouth and constipation; Nausea, vomiting, diarrhea, decreased or increased appetite, and epigastric pain may occur; very rarely - life-threatening paralytic intestinal obstruction.
From the liver and biliary tract:
Liver dysfunction (cholestatic jaundice) has been reported during antihistamine therapy.
From the skin and subcutaneous tissue:
allergic skin reactions, photosensitivity, impaired thermoregulation.
From the musculoskeletal system and connective tissue:
muscle weakness.
From the kidneys and urinary tract:
urinary disturbance.
General and administration site disorders:
nasal congestion, fatigue, lethargy.
Development of addiction:
Regular use may lead to decreased effectiveness (addiction).
Careful individual selection of the daily dose can reduce the frequency and severity of adverse reactions. The risk of adverse reactions is higher in older patients, which may increase their risk of falls.
Valocordin Doxylamine drops for oral administration 25 mg/ml 20 ml (vial-cap)
The frequency of potential adverse reactions is classified as follows:
very common (1/10), common (1/100 to <.1/10), uncommon (1/1000 to <.1/100), rare (1/10,000 to <.1/1000), very rare (up to <.1/10,000) and frequency unknown (cannot be estimated from available data).
From the blood and lymphatic system
In exceptional cases, during treatment with H1-histamin receptor blockers, the development of leukopenia, thrombocytopenia, hemolytic anemia has been reported, and very rarely cases of aplastic anemia and agranulocytosis have been reported.
From the endocrine system
In patients with pheochromocytoma, the use of H1-histamine receptor blockers may cause an increase in the release of catecholamines.
Mental disorders
Adverse reactions, depending on individual susceptibility and the dose taken, include: decreased speed
psychomotor reactions, decreased concentration, depression. In addition, paradoxical reactions may occur: restlessness, agitation, anxiety, insomnia, nightmares, confusion, hallucinations, tremor.
After prolonged daily use, abrupt cessation of therapy can lead to rebound insomnia.
Drug addiction
Like other sleeping pills, doxylamine can cause physical and mental dependence. The risk of addiction increases with increasing dose and duration of treatment, as well as in patients with alcohol or drug addiction (including a history). Rebound insomnia.
Even after short courses of doxylamine, its withdrawal can lead to a relapse of insomnia. Therefore, treatment should be stopped by gradually reducing the dose.
Anterograde amnesia
Even in therapeutic doses, doxylamine can cause anterograde amnesia, especially within the first hour after its administration. The risk increases with increasing dose, but can be reduced by sufficient uninterrupted sleep (7-8 hours).
From the nervous system
Dizziness, drowsiness, headache. In rare cases, seizures may occur.
From the side of the organ of vision
Accommodation disturbances, increased intraocular pressure.
From the organ of hearing and balance
Noise in ears.
From the cardiovascular system
Tachycardia, arrhythmia, decreased or increased blood pressure, decompensated heart failure. In several cases, ECG changes were detected.
From the respiratory organs, chest and mediastinum
Thickening of bronchial secretions, bronchial obstruction, bronchospasm, which can lead to impaired pulmonary function.
From the gastrointestinal tract
Autonomic adverse reactions, such as: dry mouth and constipation, nausea, vomiting, diarrhea, decreased or increased appetite, and epigastric pain may occur. In very rare cases, life-threatening paralytic ileus.
From the liver and biliary tract
Liver dysfunction (cholestatic jaundice) has been reported during antihistamine therapy.
From the skin and subcutaneous tissue
Skin allergic reactions, photosensitivity, violation of thermoregulation.
From the musculoskeletal system and connective tissue
Muscle weakness.
From the kidneys and urinary tract
Urinary dysfunction.
General and administration site disorders
Nasal congestion, fatigue, lethargy.
Development of addiction
Regular use may lead to decreased effectiveness (addiction).
Notes
Careful individual selection of the daily dose can reduce the frequency and severity of adverse reactions. The risk of adverse reactions is higher in older patients, which may increase their risk of falls.
VALOCORDIN-DOXYLAMINE drops internal. fl. 20 ml
Side effects
The frequency of potential adverse reactions is classified as follows:
very common (1/10), common (1/100 to <.1/10), uncommon (1/1000 to <.1/100), rare (1/10,000 to <. .1/1000), very rare (up to <.1/10,000) and frequency unknown (cannot be estimated from available data).
From the blood and lymphatic system
In exceptional cases, during treatment with H1-histamin receptor blockers, the development of leukopenia, thrombocytopenia, hemolytic anemia has been reported, and very rarely cases of aplastic anemia and agranulocytosis have been reported.
From the endocrine system
In patients with pheochromocytoma, the use of H1-histamine receptor blockers may cause an increase in the release of catecholamines.
Mental disorders
Adverse reactions, depending on individual susceptibility and the dose taken, include: decreased speed
psychomotor reactions, decreased concentration, depression. In addition, paradoxical reactions may occur: restlessness, agitation, anxiety, insomnia, nightmares, confusion, hallucinations, tremor.
After prolonged daily use, abrupt cessation of therapy can lead to rebound insomnia.
Drug addiction
Like other sleeping pills, doxylamine can cause physical and mental dependence. The risk of addiction increases with increasing dose and duration of treatment, as well as in patients with alcohol or drug addiction (including a history). Rebound insomnia.
Even after short courses of doxylamine, its withdrawal can lead to a relapse of insomnia. Therefore, treatment should be stopped by gradually reducing the dose.
Anterograde amnesia
Even in therapeutic doses, doxylamine can cause anterograde amnesia, especially within the first hour after its administration. The risk increases with increasing dose, but can be reduced by sufficient uninterrupted sleep (7-8 hours).
From the nervous system
Dizziness, drowsiness, headache. In rare cases, seizures may occur.
From the side of the organ of vision
Accommodation disturbances, increased intraocular pressure.
From the organ of hearing and balance
Noise in ears.
From the cardiovascular system
Tachycardia, arrhythmia, decreased or increased blood pressure, decompensated heart failure. In several cases, ECG changes were detected.
From the respiratory organs, chest and mediastinum
Thickening of bronchial secretions, bronchial obstruction, bronchospasm, which can lead to impaired pulmonary function.
From the gastrointestinal tract
Autonomic adverse reactions, such as: dry mouth and constipation, nausea, vomiting, diarrhea, decreased or increased appetite, and epigastric pain may occur. In very rare cases, life-threatening paralytic ileus.
From the liver and biliary tract
Liver dysfunction (cholestatic jaundice) has been reported during antihistamine therapy.
From the skin and subcutaneous tissue
Skin allergic reactions, photosensitivity, violation of thermoregulation.
From the musculoskeletal system and connective tissue
Muscle weakness.
From the kidneys and urinary tract
Urinary dysfunction.
General and administration site disorders
Nasal congestion, fatigue, lethargy.
Development of addiction
Regular use may lead to decreased effectiveness (addiction).
Notes
Careful individual selection of the daily dose can reduce the frequency and severity of adverse reactions. The risk of adverse reactions is higher in older patients, which may increase their risk of falls.