Albendazole has a wide spectrum of antiparasitic (anthelminthic) action. The drug acts selectively, inhibiting the polymerization of betatubulin, and has an ovicidal effect. Active against adults and larvae of cestodes and nematodes, sexually mature trematodes. Allows you to disinfect pastures by destroying helminth eggs.
The drug disrupts microtubular function, interferes with glucose transport, has an inhibitory effect on fumarate reductase in helminths, disrupts muscle innervation and the permeability of cell membranes, which leads to paralysis and death of helminths. Albendazole affects biochemical processes, blocks the movement of secretory granules and other organelles in the muscle cells of roundworms, is effective against larval forms of cestodes Echinococcus granulosus
,
Taenia solium
, nematodes -
Strongyloides stercolatis
.
When administered orally, it is absorbed into the gastrointestinal tract and penetrates into tissues and organs. It is not detected in blood plasma, because it is quickly transformed in the liver into the primary metabolite - albendazole sulfoxide, characterized by anthelmintic activity. Excretion of the substance occurs primarily in bile or urine, unchanged or as metabolites.
At recommended doses, Albendazole is well tolerated by animals and has no sensitizing or hepatotoxic effects.
Indications
For therapeutic and prophylactic purposes for deworming small and large livestock with:
- nematodes - ascariasis, microsporidiosis, hookworm disease, enterobiasis, trichuriasis, strongyloidiasis, opisthorchiasis, giardiasis;
- cestodias;
- trematodes.
The drug is effective against neurocysticercosis - against the larval form of pork tapeworm ( Taenia solium
). It is prescribed for mixed helminthiases, and also as an adjuvant in the surgical removal of echinococcal cysts.
Medications
As of 05.2020, 23 albendazole medicinal products with different trade names were registered in the Russian Federation. You can buy albendazole in various dosage forms, for example:
- Alben S
- (Produced by NVC Agrovetzashchita LLC, Russia, 129329, Moscow) - a combined preparation of albendazole (250 mg) and praziquantel (25 mg) in the form of tablets for oral use. Prescribed to dogs and cats for therapeutic and prophylactic purposes for nematodes (toxocariasis, toxacaridiasis, uncinariasis, hookworm disease), cestodiasis (taeniasis, dipylidiasis, echinococcosis, diphyllobothriasis, mesocestoidosis) and giardiasis. - HELMICIDE tablets
- (Produced by NVC Agrovetzashchita LLC, Russia, 1229329, Moscow) - a combined preparation of albendazole (360 mg) and oxyclozanide (175 mg) in the form of tablets for oral administration. HELMICIDE tablets are used for deworming large and small cattle with the following diseases: trematodes (acute and chronic fascioliasis, paramphistomiasis, dicroceliosis); nematodes of the gastrointestinal tract (haemonchiasis, esophagostomiasis, nematodirosis, bunostomiasis, stertagiasis, habertiosis, cooperiosis, strongyloidiasis, trichostrongylosis, trichocephalosis, neoascariasis); nematodes of the lungs (dictyocaulosis, protostrongylidosis, mulleriosis, cystocaulosis); cestodoses (moniesiosis, avitellinosis, tyzaniesiosis). - Closalben 10 and 20
- (Produced by VIK - Animal Health LLC, Russia, 140050, Moscow region, Lyubertsy) - a combined preparation of closantel (50 mg or 100 mg, respectively) and albendazole (50 mg or 100 mg, respectively) in powder form for oral use. Prescribed to cattle, sheep, goats, deer for fascioliasis, moniesiosis, edemagenosis, dictyocaulosis, ostertagiasis, hemonchiosis, dicroceliosis, trichostrongylosis, cooperiosis, esophagostomosis, as well as for cattle hypodermatosis, estrosis and psoroptosis in sheep. Closalben 10 and 20 are approved for use during pregnancy in animals. - Alvet
- (Produced by LLC NITA-PHARM, 410010, Saratov) - single-drug albendazole (200 mg) in the form of granules. Prescribed for therapeutic and prophylactic purposes to cattle, sheep, horses, pigs, dogs and poultry for nematodes, cestodes, trematodes. - Albendazole 10% granulate
- (Produced by Domestic LLC NPK Askont+, Moscow region, Serpukhov district, Obolensk) - a single preparation of albendazole 10% in the form of granular powder for oral use for deworming farm animals and birds. Prescribed for nematodes, trematodes, cestodes. - Albendazole suspension
- (Produced by LLC NPP "Agropharm"; Russia, 394061, Voronezh region, Voronezh) - a single drug of albendazole in the form of a suspension for oral use, available in two concentrations of 2.5% and 10%. Albendazole suspension 2.5% is used in cattle, goats and sheep, and Albendazole suspension 10% is used in cattle for therapeutic and prophylactic purposes for nematodes, tremotodiases and cestodiases, incl. bunostomosis, cooperiosis, hemonchosis, nematodirosis, esophagostomosis, ostertagiasis, strongyloidiasis, trichostrongylosis, habertiosis, dictyocaulosis, protostrongylosis, mulleriosis, moniesiosis, avitellinosis and chronic fasciolosis. - Febtal®-combo
- (manufactured by Otechestvenny LLC "AVZ S-P", Sergiev Posad) - a combined anthelmintic drug of praziquantel (5 mg) and albendazole (50 mg) in the form of a suspension for oral use for nematodes and cestodiases (toxocariases, toxascariasis , uncinariasis, trichocephalosis, hookworm disease, taeniasis, dipylidiasis, echinococcosis, diphyllobothriasis, mesocestoidosis) and associative nematodo-cestodiasis infestations of dogs and cats. Febtal Combo should not be used by pregnant or lactating animals, as well as puppies and kittens under 3 weeks of age. - REPTILIFE suspension
- (Produced by NVC Agrovetzashchita LLC, Russia, 1229329, Moscow) - a combined preparation of albendazole (25 mg) and praziquantel (10 mg) in the form of a suspension for oral use. REPTILIFE suspension is prescribed to turtles, lizards and snakes for prophylactic and therapeutic purposes against nematodes and cestodiases. Should not be used during laying or pregnancy (viviparous reptiles).
Recommended storage conditions for the drugs are in sealed manufacturer's packaging, in a place protected from light, at a temperature of no more than 25℃. More accurate information is provided on the packaging of drug manufacturers.
Doses and method of administration
Alben S
Apply to dogs and cats individually, orally (orally). For helminthiasis, a single dose of 1 tablet per 5 kg of animal weight in the morning feeding with a small amount of food. For giardiasis and helminthiasis complicated by giardiasis, Alben S is used in a dose of 1 tablet per 10 kg of animal weight once a day for 5 days in a row.
HELMICIDE
tablets are administered to animals individually once orally (orally) in the following doses (number of tablets per animal weight):
- for fascioliasis, dicroceliosis, paramphistomiasis and ostertagiasis: for cattle - 1 tablet per 35 kg, for sheep and goats - 1 tablet per 45 kg;
- for cestodiases and nematodes (excluding ostertagiasis): for cattle - 1 tablet per 50 kg, for sheep and goats - 1 tablet per 70 kg.
Closalben 10 and 20
used for helminthiases and gadfly infestations individually or in groups once. The drug is administered as a mixture with food or force-fed in the form of an aqueous suspension from a bottle. For psoroptosis in sheep, Closalben 10 and 20 are used twice with an interval of 7 days, and for prophylactic purposes - once.
- for gadfly infestations and helminthiasis, Closalben 20 at the rate of 40 mg per 1 kg of animal weight, and Closalben 10 at the rate of 80 mg per 1 kg of animal weight.
- for sheep psoroptosis: Closalben 20 – 100 mg/kg animal weight, and Closalben 10 – 200 mg/kg animal weight.
Alvet
administered orally individually or in a group method once, adding to the feed of cattle, sheep, pigs, horses and dogs, and poultry - twice. For group administration, a portion of the drug for a group of no more than 150 sheep is thoroughly mixed with feed (at the rate of 50-100 g of feed per animal).
- for monienosis, pulmonary and gastrointestinal nematodes: for cattle - at a dose of 3.75 g per 100 kg of animal weight (7.5 mg/kg according to the DV), sheep - 2.5 g per 100 kg of animal weight (5.0 mg/kg according to DV);
- for chronic fascioliasis: for cattle - at a dose of 5.0 g per 100 kg of animal weight (10.0 mg/kg according to DV), for sheep - at a dose of 3.75 g per 100 kg of animal weight (7.5 mg/kg according to DV);
- for horses with parascariasis, strongylidosis (alfortiosis, delafondiosis), anoplocephalosis and cyathostomosis, use individually at a dose of 3.75 g per 100 kg of animal weight (7.5 mg/kg according to the DV);
- in pigs, the drug is used for ascariasis and esophagostomiasis in a group method with concentrated feed in the morning feeding at a dose of 5.0 g per 100 kg of animal weight (10.0 mg/kg according to the DV). The drug, in a dose designed for a group of no more than 50 pigs, is mixed with half the daily feed intake;
- for dogs, the drug is used individually for nematodes at a dose of 0.375 g per 10 kg of animal weight (7.5 mg/kg according to the DV), for cestodes at a dose of 0.75 g per 10 kg of animal weight (15 mg/kg according to the DV);
- For farm poultry, the drug is used for ascariasis and heterokidosis for 2 days in a row in a group method - by adding it to the mixed feed in the morning feeding at a dose of 0.5 g per 10 kg of bird weight (10.0 mg/kg according to the DV).
Albendazole 10%
The granulate is used individually or in groups mixed with food. Cattle, sheep, horses and poultry twice. The drug is administered in the following doses:
- for gastrointestinal and pulmonary nematodes of cattle - 75 mg/kg body weight; sheep - 50 mg/kg body weight. When administering albendazole to sheep in a group, the drug is dosed per group of no more than 150 heads, mixing it with feed - 50-100 g of feed per individual;
- for chronic fascioliasis in cattle - 100 mg/kg body weight; sheep - 75 mg/kg body weight;
- for esophagostomiasis and ascariasis in pigs, the drug is mixed with half the daily norm of concentrated feed at a dose of 100 mg/kg of animal weight. Medicinal food is created for a group of no more than 50 animals and is introduced into the morning feeding;
- for anoplocephalidosis and cyathostomosis, parascariasis, strongyliasis, delafondiosis, alfortiosis, horses, the drug is administered individually - 75 mc/kg body weight;
- for ascariasis, heteracidosis and mixed ascariasis-heteracidosis, birds choose the group method. According to the instructions for use of albendazole, the medicine is administered in mixture with feed for 2 days in a row in the morning at a daily dose of 100 mg/kg of bird weight.
Albendazole
the suspension is administered once, individually, orally using a syringe dispenser:
- for moniesiosis, gastrointestinal and pulmonary nematodes in cattle - 3 ml per 10 kg (7.5 mg/kg weight according to the DV), small cattle - 2 ml per 10 kg of weight (5 mg/kg weight according to the DV) ;
- in the treatment of chronic fascioliasis in cattle - 4 ml per 10 kg of weight (10 μg of weight according to the DV), small cattle - 3 ml per 10 kg of weight (7.5 mg/kg of weight according to the DV);
- for esophagostomiasis and ascariasis in pigs - 2 ml per 10 kg of weight (5 mg/kg of weight according to the DV).
Febtal®-combo
applied to animals individually, once in the morning feeding with a small amount of food or forcefully on the root of the tongue, the dose is calculated as 1 ml of suspension per 1 kg of weight.
REPTILIFE suspension
. The drug is used individually, orally (orally) using a dosing syringe at the rate of 1 ml of suspension per 1 kg of reptile weight, 2-3 times with an interval of 14 days.
Nemozol suspension for oral administration 100 mg/5 ml 20 ml No. 1
Name
Nemozol.
Release form
Oral suspension
Dosage
100 mg / 5 ml 20 ml. Packing quantity: 1 pc.
Manufacturer
Ipka Laboratories Ltd.
INN
Albendazole.
FTG
Anthelmintic and antiprotozoal agent.
Compound
The oral suspension contains: active substance: albendazole 100 mg in 5 ml excipients: microcrystalline cellulose and carboxymethylcellulose sodium salt, carboxymethylcellulose sodium salt, glycerin, benzoic acid, potassium hydroxide, sorbic acid, polysorbate-80, sorbitol solution, mixed fruit essence , flavoring, ice cream essence, purified water.
Description
Suspension for oral administration White to almost white suspension. Delamination is allowed, which is eliminated by shaking.
Pharmacotherapeutic group
Anthelmintics. Means for the treatment of nematodes. ATX code: P02CA03.
pharmachologic effect
The main mechanism of action of albendazole is its inhibitory effect on beta-tubulin polymerization, which leads to the destruction of cytoplasmic microtubules of helminth intestinal tract cells; changes the course of biochemical processes (suppresses glucose utilization), blocks the movement of secretory granules and other organelles in the muscle cells of intestinal and tissue parasites. Albendazole has larvicidal, ovicidal and anthelmintic effects, causing energy depletion of helminths, which leads to their immobilization and destruction.
Pharmacokinetics
Poorly absorbed (less than 5%) in the gastrointestinal tract, unchanged form is not detected in the blood plasma, because It is quickly converted in the liver into the primary metabolite of albendazole sulfoxide, which also has anthelmintic activity. The systemic pharmacological effect of albendazole is enhanced when taken with fatty foods, which increases absorption by approximately 5 times. Absorption of albendazole sulfoxide is achieved after 2-5 hours. It is 70% bound to plasma proteins and widely distributed throughout the body; found in urine, bile, liver, cyst wall and cyst fluid, cerebrospinal fluid. Albendazole sulfoxide is converted in the liver to albendazole sulfone (a secondary metabolite) and other oxidized products. T1/2 of albendazole sulfoxide 8-12 hours. Excreted in urine. Renal excretion of albendazole and its main metabolite, albendazole sulfoxide, is insignificant; clearance does not change in patients with impaired renal function. Against the background of liver damage, bioavailability increases and Cmax of albendazole sulfoxide increases 2 times, T1/2 lengthens. Albendazole is an inducer of microsomal enzymes of the cytochrome P-450 system; accelerates the metabolism of many drugs. Pharmacokinetics in special patient groups - Extrahepatic cholestasis: Since the elimination of albendazole sulfoxide is reduced, the patient should be closely monitored. - Elderly patients: Clinical pharmacokinetic studies of albendazole sulfoxide in elderly patients have not been conducted, but data obtained from the treatment of 26 patients (age <79 years) with hydatid cysts suggest that the pharmacokinetics in this age group of patients are similar to those in young healthy volunteers. - Use in renal failure: The pharmacokinetics of albendazole in patients with renal failure have not been studied. - Use in liver failure: the pharmacokinetics of albendazole in patients with liver failure have not been studied. — Features of pharmacokinetics in children: clinical studies of the safety of albendazole in children aged 6-13 years were conducted. Albendazole was administered once to patients with echinococcosis (three children on an empty stomach and two after meals) in doses of 200 to 300 mg (at a rate of about 10 mg/kg). However, no differences were identified in the pharmacokinetics of albendazole compared to adult patients. Clinical experience with albendazole in children under 6 years of age is limited. At the same time, no data were found indicating the presence of peculiarities in the tolerability of the drug in patients of this age group who suffered from echinococcosis. There are data from five clinical studies that included 1-year-old children with neurocysticercosis. The development of significant adverse effects of albendazole was not observed. At the same time, the effectiveness of albendazole was similar to that in adult patients.
Indications for use
Intestinal helminthiasis, including mixed helminthic infestations caused by the following helminths and parasites: Nematodes: Ascaris lumbricoides (roundworm), Trichuris trichiura (whipworm), Enterobius vermicularis (pinworm), Ankylostoma duodenale (hookworm), Necator americanus (nematode), Strongyloides stercoralis (intestinal eel), hookworms that cause larval (larval) helminthiasis (skin form). Trematodes: Clonorchis sinensis (Chinese fluke), Opisthorchis viverrini (squirrel fluke). Cestodes: Taenia solium (pork tapeworm), Taenia saginata (bovine tapeworm), Hymenolepis papa (dwarf tapeworm), if they occur in combination with nematodes. If the infection occurs exclusively with tapeworms or other cestodes, then Nemozol should not be used. Protozoa: Giardia lamblia (intestinal or duodenal). Systemic helminthiases: Albendazole demonstrates the greatest effectiveness in the treatment of cysts of the liver, lungs and peritoneum. Experience with cysts of the bones, heart and central nervous system is limited. cystic echinococcosis (caused by Echinococcus granulosus). Albendazole is used to treat patients with cystic echinococcosis. if surgery is not suitable; before surgery; after surgery if preoperative treatment was too short-lived, if cyst contents leaked, or if viable material was identified during surgery; after percutaneous drainage of cysts for diagnostic or therapeutic purposes. alveolar echinococcosis (caused by Echinococcus multilocularis) Albendazole is used to treat patients with alveolar echinococcosis: in case of inoperable disease, especially in cases of local or distant metastases; after palliative surgery; after radical surgery or liver transplantation. neurocystecircosis caused by the larval form of the pork tapeworm (Taenia solium). Albendazole is used to treat patients with the following conditions: single or multiple cysts or granulomatous lesions of the brain parenchyma; arachnoid or intraventricular cysts; grape-shaped cysts. capillariasis caused by Capillaria philippinensis. Gnathostomiasis caused by Gnathostoma spinigerum. trichinosis (caused by Trichinella spiralis and T. pseudospiralis). toxocariasis (caused by Toxocara canis and other related species)
Contraindications
Hypersensitivity to the active substance or any of the auxiliary components, other benzimidazole derivatives. Albendazole should not be used during pregnancy or in women who suspect pregnancy. Women of childbearing potential should be advised to take effective contraception, including non-hormonal contraceptives, during treatment and for one month after completion of treatment.
Directions for use and doses
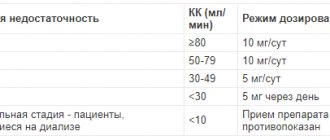
The drug is taken orally during meals. It is recommended to treat all family members simultaneously. The usual dosage for adults and children over 2 years of age (> 10 kg) for the treatment of the following helminthic infestations: Nematodes, including ascariasis, trichuriasis, enterobiasis, hookworm, necatoriasis - 400 mg of albendazole (1 tablet or 20 ml of suspension) orally once. Strongyloidiasis - 400 mg albendazole (1 tablet or 20 ml suspension) orally for 3 days. Note: The recommended dosage applies only to intestinal parasites and strongyloidiasis. The dosage may not be suitable for the treatment of patients with weakened immune systems and severe damage to internal organs. See "Special Dosage Instructions." Mixed helminthiasis (intestinal nematodes in combination with flat tapeworms) Taenia solium (pork tapeworm), Taenia saginata (bovine tapeworm) - 400 mg of albendazole (1 tablet or 20 ml suspension) orally for 3 days Hymenolepis papa (dwarf tapeworm) - 400 mg albendazole (1 tablet or 20 ml suspension) orally for 3 days, repeat the course after 2-3 weeks Trematodes caused by Clonorchis sinensis (Chinese fluke), Opisthorchis viverrini (squirrel fluke) orally 400 mg albendazole (1 tablet or 20 ml suspension ) 2 times a day for 3 days. Giardiasis (in children 2-12 years old): 400 mg of albendazole orally (1 tablet or 20 ml of suspension) for 5 days. Dosage for children 1-2 years old: half the standard dose. Use only suspension. Dosage for children over 2 years of age but weighing less than 10 kg: half the standard dose. Use only suspension. Duration of treatment See Usual Dosage. If no improvement is observed after 3 weeks: repeat treatment. Systemic helminth infections: To date, there is limited experience with the use of albendazole in high doses in children under six years of age, so use in children under six years of age is not recommended for treatment. The dosage depends on the causative parasite, the patient’s body weight, as well as the severity of the infection: Infection The patient’s body weight Dose Duration of treatment and frequency of dosing per day Cystic echinococcosis > 60 kg 800 mg in two separate doses of 400 mg Daily for 28 days. The 28-day course of therapy can be repeated after 14 days, during which the drug is not prescribed; a total of three such treatment cycles can be carried out 60 kg 60 kg 60 kg 800 mg in two equal separate doses Treatment is usually carried out from seven (minimum) to 28 days 60 kg 60 kg
Special dosage instructions
Elderly: Data on the use of the drug in patients over 65 years of age are insufficient. Anecdotal reports indicate that dosage restrictions are not required. However, elderly patients with impaired liver function should use Nemozol with caution. Use in renal failure: Since the excretion of albendazole and the original metabolite albendazole sulfoxide through the kidneys is negligible, no dosage adjustment is necessary. However, patients with renal failure require monitoring. Use in liver failure: Since albendazole is rapidly metabolized through the liver into the pharmacologically important metabolite albendazole sulfoxide, reduced liver function affects the pharmacokinetics of albendazole sulfoxide. Patients with elevated transaminase levels require monitoring of liver enzyme levels before starting treatment. Treatment with albendazole should be discontinued as soon as liver enzyme levels begin to deteriorate or if a clinically significant decrease in blood cells is observed (see Warnings and Precautions and Side Effects). Correct method of administration Young children should be treated with a suspension. The suspension (with fruit flavor) is taken undiluted or mixed with a drink. The bottle must be shaken well before use. If you accidentally miss the time of taking the drug, the next dose should be taken at the first opportunity. If the time for the next dose of the drug is approaching, the next dose should be taken according to the schedule, without increasing the total dose.
Side effects
Data from large clinical trials and post-marketing experience were used to determine the incidence of side effects. Frequency determination: very common: ≥ 10%; often: ≥ 1% and 400 mg/day) and increased duration of treatment (> 10 days), additional cases of increased intracranial pressure, neck stiffness, acute renal failure, leukopenia, pancytopenia, aplastic anemia, agranulocytosis, very often increased liver function enzymes, hepatitis, reversible alopecia and fever. Patients with liver disease, including hepatic echinococcosis, are more susceptible to bone marrow depression (see Dosage/Administration and Precautions). If the listed adverse reactions occur, as well as if other adverse reactions not described in these instructions occur, you should consult a doctor.
Interaction with other drugs
With simultaneous use of praziquantel, cimetidine or dexamethasone in the blood plasma, the concentration of the active metabolite albendazole (sulfoxide) may increase several times, which may increase the frequency of side effects. Grapefruit juice also increases blood levels of albendazole sulfoxide. Ritonavir, phenytoin, carbamazepine, phenobarbital, levamisole, ritonavir may reduce the concentration of active metabolites of albendazole in blood plasma. The clinical significance of this effect is unknown; but the effectiveness of Nemozol may decrease, in particular, with systemic helminthic infestations. In this case, the effectiveness of treatment should be monitored. Under certain circumstances, alternative dosages or treatments are necessary. Due to possible changes in the activity of cytochrome P-450, there is theoretically a risk of interaction with oral contraceptives, anticoagulants, hypoglycemic agents, theophylline. In all cases, caution should be exercised.
Precautionary measures
Nemozol suspension contains: benzoic acid, which may irritate the skin, eyes and mucous membranes. Benzoic acid may increase the risk of jaundice in newborns. In this age group (
Pregnancy and breastfeeding period
Animal studies have shown adverse effects on the fetus. Currently, there is insufficient data on the use of Nemozol in pregnant women. The drug is contraindicated in pregnant women (see “Contraindications”). If it is necessary to use the drug, women of reproductive age should use effective methods of contraception (see “Precautions”). Breastfeeding: No studies have been conducted in breastfeeding women. There is no data on whether albendazole or its metabolites are excreted in breast milk. Therefore, Nemozol can be used during breastfeeding only if the expected benefit to the mother outweighs the potential risk to the child.
Impact on the ability to drive vehicles and operate machinery
Dizziness may occur when taking albendazole. Patients should be warned about the need to take special care when driving vehicles and while working with potentially dangerous mechanisms.
Overdose
Treatment: symptomatic, gastric lavage, activated carbon.
Package
Oral suspension 100 mg / 5 ml, 20 ml each in a white plastic bottle with a screw-on aluminum cap with a gasket and tamper evident.
Best before date
Suspension for oral administration - 3 years.
Storage conditions
Store in a place protected from moisture and light at a temperature below 25°C. Keep away from children.
Vacation conditions
By doctor's prescription.
Buy Nemozol suspension d/pr. orally 100mg/5ml in vial. 20ml per pack. No. 1 in pharmacy
Price for Nemozol suspension d/pr. orally 100mg/5ml in vial. 20ml per pack. No. 1
Instructions for use for Nemozol Susp d/pr. orally 100mg/5ml in vial. 20ml per pack. No. 1
Waiting times for livestock products
For the drug Alben S there is no waiting period for livestock products.
HELMICIDE tablets – obtaining meat products is permitted no earlier than 21 days after deworming. When slaughtered earlier than the established deadline, the meat is used as feed for fur-bearing animals. Milk from dairy animals cannot be used for food purposes for 4 days after deworming. Milk obtained earlier than the established period can be used in animal feed after boiling.
Closalben 10 and 20 – production of meat products is permitted no earlier than 20 days after the end of taking the drug. Animal meat obtained before the end of the specified period is used to feed fur-bearing animals. Closalben 10 and 20 should not be given to dairy animals whose milk is intended for human consumption.
Alvet - slaughter of animals for meat is permitted no earlier than 20 days, birds - no earlier than 5 days after taking the drug. Meat from animals killed before the end of this period can be used as feed for fur-bearing animals. Milk from dairy animals must not be consumed for 4 days after taking Alvet. Milk obtained before the expiration of this period, after heat treatment, can be used to feed animals.
Albendazole 10% granulate - obtaining animal meat is allowed no earlier than 20 days, poultry meat - no earlier than 5 days after taking the drug. Meat products received before the end of the specified period are permitted for use as feed for fur-bearing animals. Milk can be used for food no earlier than 4 days after administration of the drug to dairy animals. Before this period expires, milk can be used as animal feed after boiling.
Albendazole suspension - slaughter of animals for meat after taking the drug is permitted no earlier than 20 days. Meat from animals killed ahead of schedule is allowed to be used to feed fur-bearing animals. Milk from dairy animals cannot be used for food purposes within 7 days after taking Albendazole suspension. Milk obtained earlier than the established deadline can be used as animal feed after heat treatment.
There are no waiting periods for the drug Febtal®-combo for livestock products.
REPTILIFE suspension is not intended for use in productive animals.
I. General information
1. Trade name of the drug: Albendazole 100 (Albendasol 100).
International nonproprietary name: albendazole.
2. Dosage form: suspension for oral use.
Albendazole 100 as an active ingredient in 1 ml contains 100 mg of albendazole, and as auxiliary components - 1 mg of polysorbate 80, 1 mg of benzoic acid, 1 mg of fellow potassium, 5 mg of carboxymethylcellulose, 60 mg of glycerin and water for injection up to 1 ml. In appearance, the drug is a suspension from milky white to light gray.
3. Albendazole 100 is produced in 1,000 ml polyethylene bottles with screw caps.
4. Store the medicinal product in the manufacturer’s sealed packaging, in a dry place, protected from direct sunlight, separately from food and feed, at a temperature of 5°C to 25°C.
The shelf life of the medicinal product, subject to storage conditions in the manufacturer's closed packaging, is 2 years from the date of production. Do not use Albendazole 100 after the expiration date.
5. Albendazole 100 should be kept out of the reach of children.
6. Unused medicinal product is disposed of in accordance with legal requirements.
II. Pharmacological properties
7. Albendazole 100 is a broad-spectrum anthelmintic drug.
Albendazole, which is part of the drug, has a wide spectrum of anthelmintic action, is active against the adults and larvae of nematodes, trematodes, as well as sexually mature forms of cestodes; has an ovicidal effect, which reduces the infestation of pastures with helminth eggs.
The mechanism of action of albendazole is to disrupt the processes of glucose transport, microtubular function and reduce the activity of helminth fumarate reductase, disrupt the permeability of cell membranes and muscle innervation, which causes paralysis and death of parasites.
When administered orally, albendazole is absorbed from the gastrointestinal tract and penetrates into organs and tissues; the maximum concentration in blood serum is observed 18-25 hours after application. Albendazole is excreted from the body mainly in urine and bile in unchanged form and in the form of metabolites.
In terms of the degree of impact on the body, Albendazole 100 is classified as a moderately hazardous substance (hazard class 3 according to GOST 12.1.007-76).
III. Application procedure
8. Albendazole 100 is used in cattle, sheep, goats and pigs for the treatment and prevention of nematodes, trematodes and cestodiases, including dictyocaulosis, protostrongyliasis, hemonchiasis, ostertagiasis, trichostrongyliasis, bunostomosis, nematodirosis, cooperiosis, esophagostomiasis, habertiosis, moniesiosis, a also chronic fascioliasis.
9. The use of Albendazole 100 is not allowed for dicraceliosis and acute form of fascioliasis, during the hunting period, pregnant cows in the first third of pregnancy, pregnant sheep, goats and pregnant sows in the first half of pregnancy, as well as patients with infectious diseases and emaciated animals.
10. Albendazole 100 is administered to animals once orally, in the following doses:
— for cattle with moniesiosis, pulmonary and gastrointestinal nematodes – 0.75 ml per 10 kg of animal weight (7.5 mg per 1 kg of animal weight according to the DV); for chronic fascioliasis - 1 ml per 10 kg of animal weight (10 mg per 1 kg of animal weight according to the DV);
— sheep and goats for moniesiosis, pulmonary and gastrointestinal nematodes – 0.5 ml per 10 kg of animal weight (5 mg per 1 kg of animal weight according to the DV); for chronic fascioliasis - 0.75 ml per 10 kg of animal weight (7.5 mg per 1 kg of animal weight according to the DV);
- pigs with ascariasis and esophagostomosis - 0.5 ml per 10 kg of animal weight (5 mg per 1 kg of animal weight according to the DV).
Before use, the drug in the vial must be shaken thoroughly.
Before mass treatments, each series of Albendazole 100 is preliminarily tested on a small group of animals (10-15 animals), which are monitored for 3 days. In the absence of side effects, the drug is administered to the entire livestock.
11. In case of an overdose of the drug, the animal may experience anorexia, impaired coordination of movement, and lethargy. In this case, the use of the drug is stopped and symptomatic treatment is prescribed.
12. The specific effects of the drug upon first taking the drug and upon its withdrawal have not been established.
13. The drug is used once. Animals do not require a special diet or use of laxatives before deworming.
14. As a rule, there are no side effects or complications when using Albendazole 100 in accordance with these instructions. If allergic reactions occur, stop using the drug and prescribe the animal antihistamines and symptomatic therapy.
15. Albendazole 100 should not be used simultaneously with other antiparasitic drugs, as well as dexamethasone and cimetidine.
16. Slaughter of animals for meat is permitted no earlier than 20 days after the use of Albendazole 100. Meat of animals forcedly killed before the expiration of the specified period can be used as feed for fur-bearing animals. Milk from dairy cows is allowed to be used for food purposes no earlier than 7 days after the last administration of the drug. Milk obtained earlier than the specified period can be used as animal feed after boiling.
IV. Personal prevention measures
17. When working with Albendazole 100, you should follow the general rules of personal hygiene and safety precautions provided for when working with medications. After finishing work, wash your hands with warm water and soap.
18. In case of accidental contact of the drug with the skin or mucous membranes of the eyes, they must be rinsed with plenty of water. People with hypersensitivity to the components of the drug should avoid direct contact with Albendazole 100. In case of allergic reactions or if the drug accidentally enters the human body, you should immediately contact a medical facility (have instructions for use of the drug or label with you).
19. Empty medicinal product containers must not be used for household purposes; they must be disposed of with household waste.
Place of manufacture: Wuqi Intersection, Shijiazhuang, Hebei Province, 050041, China.
The instructions were developed by Gordonos, 7B, Agios Georgios Chavouzas, PC 3070, Limassol, Cyprus in collaboration with Hebei Yuanzheng Pharmaceutical Co., Ltd., Wuqi Intersection, Shijiazhuang, Hebei Province, 050041, China.