Home | About us | Delivery | Advertisers | Login | Registration
The pharmacy is closed on Sundays and holidays.
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2021 Pharmacy 84.
Flurbiprofen: new possibilities for local treatment of tonsillopharyngitis
A.A. Zaitsev, O.I. Karpov, S.A. Karpishchenko
Flurbiprofen is considered one of the quickly and effectively acting non-steroidal anti-inflammatory drugs (NSAIDs), which have been successfully used for many years to treat joint syndromes of various etiologies. It is characterized by a wide range of anti-inflammatory and analgesic effects when used in moderate therapeutic dosages [1].
At the same time, it should be recognized that NSAIDs that are more selective with respect to their effects on cyclooxygenase type 2 (COX-2) are gradually replacing non-selective drugs due to better tolerability and fewer complications when long-term use is necessary. On the other hand, the anti-inflammatory effect of selective NSAIDs develops somewhat more slowly, which in some cases makes it problematic to solve the problem of acute pain syndrome arising, for example, due to infectious inflammation [2]. A practical solution to this problem, as it turns out, lies in the use of effective non-selective NSAIDs locally [3]. In this case, it is possible to reduce the dosage, there is no resorptive effect, and therefore the safety of pharmacotherapy increases.
It turned out that flurbiprofen, more than other NSAIDs, satisfies the conditions for local effects on inflammation, which was previously demonstrated when using its external forms in rheumatological and ophthalmological practice. When applied topically, the therapeutic effect occurred so quickly, and resorption from the surface of the skin and conjunctiva was so insignificant that systemic manifestations of action or adverse reactions were not observed [4,5]. Clinical and pharmacological studies of flurbiprofen performed in patients with acute sore throat, the main cause of which is considered to be a viral infection, showed that the symptomatic effect of the drug can be very useful in improving the quality of treatment.
An important aspect is that the rapid positive dynamics provided by flurbiprofen allows one to avoid the unjustified empirical use of antibiotics. At the same time, we emphasize once again that the dosage of the drug is only 8.75 mg, which is enough to develop the anti-inflammatory and associated analgesic effect within 15–30 minutes and maintain them for several hours [6]. In 2000–2001 results of the use of a dosage form of flurbiprofen for resorption (Strepfen) were published.
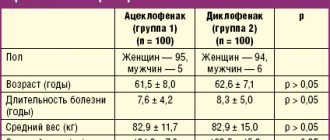
Based on double-blind, placebo-controlled, randomized studies involving hundreds of patients, it was concluded that this drug is highly clinically effective and safe. The most revealing results of studies by Benrimoj SI et al. (2000) [7] and Warson N. et al. (2000) [8]. After taking the first dose, a statistically significant analgesic effect occurred within 15 minutes, lasting at least 2 hours. Subsequent administration of the drug made it possible to achieve a pronounced reduction in symptoms in the majority of patients - pain and swelling in the throat on days 2–3; in the group of patients receiving placebo, these manifestations of the disease disappeared at a later date. We obtained clinical evidence of the effectiveness of flurbiprofen in an open study in patients with acute viral tonsillopharyngitis. Patients with a duration of symptoms of no more than 3 days took part in it and signed informed consent. The study did not include patients with: – intolerance to NSAIDs; – bronchial asthma; – peptic ulcer of the stomach and duodenum; – pneumonia, bronchitis; – who have received antimicrobial agents or drugs with antiseptic, anesthetic and anti-inflammatory effects for this disease; – pregnant and lactating women; – severe concomitant diseases of the cardiovascular, hepato-biliary and urinary systems; – stomatitis as a result of cytostatic therapy; – rheumatic diseases. Strepfen was used 1 tablet every 3–6 hours (but not more than 5 tablets per day) for 3 days. The drug dissolved in the mouth. All patients received comprehensive instructions on the use of the drug. Efficacy was assessed by reducing the severity of sore throat, difficulty swallowing and inflammation using a visual analogue scale (VAS). In this case, the absence of a sign was taken as 0, and its maximum severity was taken as 10. Patients also assessed the overall effect of treatment, for which an inverted VAS scale (countdown) was established - 10 - no effect, 0 - maximum effect. During patient visits, the researcher also assessed hyperemia (on a 5-point scale), swelling of the pharynx (on a 3-point scale) and enanthema (on a 3-point scale), the number, density, size of the cervical and mandibular lymph nodes. The safety of use was assessed based on taking into account adverse events that could occur while taking the drug. Tolerability was assessed as good in the absence of side effects, satisfactory in the presence of adverse reactions associated with taking the drug but not requiring therapeutic intervention, unsatisfactory in the presence of adverse reactions to the drug requiring additional therapeutic intervention. The study included 20 patients (9 men and 11 women). Average age – 36.9±5.9 years (minimum age – 19 years, maximum – 62 years). The duration of the disease in most patients (11 people) was 2 days, in the rest – 3 days. Since the first dose of the drug was taken by different patients at different times, the following VAS time points were taken to analyze subjective sensations: – 1–2 hours after the first dose of the drug; – 21:00 on the first day of admission; – 9 a.m. and 3 p.m. on the second day of treatment; – 9 a.m. and 9 p.m. on the third day of treatment; – 9 o’clock on the fourth day.
The data obtained confirm the high effectiveness of Strepfen as an anti-inflammatory agent. Within 1–2 hours after taking the drug, the reduction in sore throat and difficulty swallowing, as assessed by VAS, occurred by an average of 33–35%, and by the end of the first day of use, the VAS score “Sore throat” decreased by more than half (Fig. 1), and “Difficulty in swallowing” – 3 times. A decrease in the “Sore throat” indicator by 50% or more 1–2 hours after taking the drug was recorded in 8 out of 20 patients, and by the end of the 1st day of treatment – in another 6 people (14 patients in total), and in 4 patients VAS values were minimal (20–30%). Strepfen had a somewhat more pronounced effect on swallowing. Difficulty in swallowing, which was initially present in all patients, by the end of the 1st day of treatment decreased significantly (by 50% or more) in 8 patients, and 5 patients by this time no longer experienced difficulty swallowing. Thus, clear positive dynamics were noted already during the first day of treatment. The decrease in the severity of clinical signs of pharyngitis continued during the 2nd day of treatment and reached its minimum values on the 3rd day of treatment. Most patients took Strepfen for 3 days (17 people), 3 patients were treated for 2 days. The dynamics of inflammation assessed by VAS practically repeats the two previous indicators - the most significant subjective feeling of a decrease in inflammation was on the first day of taking the drug (by 56.6% by the end of the day), a further natural decrease was observed on the subsequent day (by an average of another 23.9%) . On the 3rd day, the inflammation in the throat was practically not felt by the patients, which is confirmed by objective data from the examination of the patients. Hyperemia of the pharynx decreased by more than half on the 2nd day; on the 3rd day of treatment it was not present in 3 patients, and in 13 people the manifestations were minimal (1 point on the researcher’s scale). On the 4th day, there was no hyperemia of the pharynx in all 20 patients. Swelling of the pharynx disappeared on the 2nd day in 3 patients, and in another 9 on the 3rd day. In the remaining 8 patients, when examined on the 3rd day, it was weakly expressed, and on the 4th day it was not detected. The overall effect of treatment was noticeable already within the 1st day of treatment, as evidenced by the average VAS scores (an increase of 53.8%) (Fig. 2).
By the end of 3 days, all patients rated the effect of the treatment as 100%. During treatment, in no case was an undesirable effect of Strepfen recorded. Therefore, the tolerability of the drug can be assessed as good. Our results coincide with the data of other authors. It is especially important that there is evidence of a rapid reduction in pain when swallowing. The drug more clearly demonstrates the process of reducing inflammation, since it ensures the subsidence of painful manifestations not only in the superficial, but also in the deep layers of the pharyngeal mucosa. In conclusion, we note that the pharmaceutical industry offers many remedies for the treatment of sore throats - from herbal to medicinal with the addition of either antiseptic, antimicrobial, or local anesthetic components. In our opinion, treatment aimed at suppressing the inflammatory reaction in the oropharynx can only be effective with the help of NSAIDs that act on the key link in maintaining this reaction. However, some caution should be exercised when prescribing flurbiprofen (even topically) to patients with a history of peptic ulcers, bronchial asthma, hemophilia, elderly patients, as well as non-ulcer dyspepsia, since some part of the drug can be swallowed and have an effect directly in the digestive organs. Due to the lack of safety data, it is not recommended to use Strepfen in children. Thus, Strepfen, a drug with targeted (local) anti-inflammatory action, demonstrates high clinical efficacy, good tolerability and can be used for the treatment of acute viral tonsillopharyngitis.
Literature
1. Davies NM Clinical pharmacokinetics of flurbiprofen and its enantiomers // Clin. Pharmacokinet. – 1995. – Vol.28. – No.2. – P.100–114.
2. Phero JC, Becker DE, Dionne RA et al. Contemporary trends in acute pain management // Curr. Opin. Otolaryngol. Head. Neck. Surg. – 2004. – Vol.12. – No.3. – P.209–216.
3. Devillier P. Pharmacology of non-steroidal anti-inflammatory drugs and ENT pathology // Presse. Med. – 2001. – Vol.30. – No.39–40 (Pt. 2). P.70–79.
4. Droge MJ, van Sorge AA, van Haeringen NJ et al. Alternative splicing of cyclooxygenase-1 mRNA in the human iris // Ophthalmic. Res. – 2003. – Vol.35. – No.3. – P.160–163.
5. Fang JY, Hwang TL, Fang CL, Chiu HC In vitro and in vivo evaluations of the efficacy and safety of skin permeation enhancers using flurbiprofen as a model drug // Int. J. Pharm. – 2003. – Vol.255. – No.1–2. – P.153–166.
6. Benrimoj SI, Langford JH, Homan HD et al. Efficacy and safety of the anti-inflammatory throat lozange flurbiprofen 8.75 mg in the treatmant of sore throat // Fundament. Clin. Pharmacol. – 1999. – Vol.13. – P.189.
7. Benrimoj SI et al. Efficacy and tolerability of the anti-inflammatory throat lozenge flurbiprofen 8.75 mg in the treatment of sore throat – a randomized, double-blind, placebo-controlled study // Clin. Drug. Invest. – 2001. – Vol.21. – No.3. – P.183–193.
8. Warson N., Nimmo WS, Christian J. et al. Relief of sore throat with the anti-inflammatory throat lozenge flurbiprofen 8.75 mg: a randomized, double-blind, placebo-controlled study of efficacy and safety // Int. J. Clin. Pract. – 2000. – Vol.54. – No.8. – P.490–496.
Published with permission from the administration of the Russian Medical Journal.