Chemical properties
Alfacalcidol is an analogue of vitamin D , which is actively used as an additive to food and poultry feed to replenish vitamin reserves. The drug is included in the list of vital and essential drugs.
The medicine is synthesized in the form of white or colorless crystals, which are practically insoluble in water, but easily soluble in ethanol , and slowly dissolve in vegetable oil. This chemical compound is not stable and decomposes under the influence of ultraviolet radiation and air. Molar mass of the substance = 400.6 grams per mole.
Pharmacodynamics and pharmacokinetics
The medicine plays an important role in the processes of maintaining the homeostasis of phosphorus and calcium metabolism . Alfacalcidol stimulates the active absorption of phosphates and calcium from the lumens of the small intestine and increases the degree of calcium reabsorption in the renal tubules. The drug increases the intensity of bone tissue mineralization, reduces the degree of osteomalacia and the level of parathyroid hormone . The drug prevents rickets .
This chemical compound reduces the incidence of fractures resulting from calcium imbalance due to calcium malabsorption . The patient's coordination of movements improves, pain in the bones and muscles disappears.
After taking the drug, the effect of the tablets develops within 6 hours and lasts about 2 days. Alfacalcidol is absorbed in the small intestine. The maximum concentration of the drug in plasma is observed after 8-18 hours. The half-life from the blood is about 3 hours. Metabolism occurs in the liver, where the active metabolite 1,25-dihydroxycolecalciferol is formed; to a lesser extent, the substance is metabolized in bone tissue. Metabolites are excreted in bile and urine.
Due to the fact that the drug is not metabolized in the kidneys, it can be prescribed for varying degrees of renal failure.
Pharmacological properties of the drug Alfacalcidol
Regulator of calcium and phosphorus metabolism in the body. Increases the absorption of calcium and phosphorus in the intestines, enhances their reabsorption in the kidneys, increases bone mineralization, and reduces the level of parathyroid hormone in the blood. Restores a positive calcium balance during malabsorption, reduces the severity of calcium resorption from bones and reduces the incidence of fractures due to osteoporosis. With a course of use, there is a decrease in pain in the bones associated with impaired phosphorus-calcium metabolism, and in the muscles, and coordination of movements improves. After oral administration, it is quickly absorbed from the digestive tract. In the liver, alfacalcidol is rapidly metabolized to form a pharmacologically active metabolite, calcitriol (1,25-dihydroxyvitamin D3); a smaller part of the active substance is metabolized in bone tissue. Unlike natural vitamin D, alfacalcidol is not biotransformed in the kidneys, which allows its use in patients with impaired renal function.
Indications for use
The medicine is prescribed:
- as a prophylactic for hypo- or vitamin deficiency D ;
- patients with osteomalacia , osteopathy , osteoporosis ;
- with unbalanced and inadequate nutrition;
- patients with hypoparathyroidism with hypocalcemia ;
- with malabsorption and insufficient insolation of vitamins ;
- patients with vitamin D-resistant rickets ;
- alcoholics;
- for liver cirrhosis or liver failure, as part of complex therapy;
- patients with Fanconi syndrome ;
- with obstructive jaundice ;
- if some gastrointestinal diseases interfere with the absorption of vitamin D ( persistent diarrhea , Crohn's disease , celiac disease , tropical sprue );
- with rapid weight loss;
- pregnant women, especially with multiple pregnancies, dependence on nicotine or certain drugs;
- with insufficient insolation of breastfed newborns.
Contraindications
The drug is contraindicated for use:
- patients with hypercalcemia ;
- if allergic to the active substance;
- patients with renal osteodystrophy with hyperphosphatemia ;
- with hypervitaminosis D.
Particular caution must be observed (treatment is carried out under the supervision and recommendation of a doctor):
- for atherosclerosis ;
- patients with active pulmonary tuberculosis
- patients with sarcoidosis and other granulomatosis ;
- with heart failure;
- for chronic kidney diseases;
- if the patient has a history of nephrourolithiasis ;
- with hyperphosphatemia ;
- elderly patients, to avoid the development of atherosclerosis ;
- children.
Side effects
Usually the medicine does not cause any adverse reactions.
May appear:
- anorexia , nausea, discomfort in the epigastric region, vomiting, dryness of the oral mucosa, constipation ;
- increased fatigue, drowsiness , weakness, dizziness;
- skin rashes, allergic itching.
Rarely occur:
- increased activity of liver enzymes, ALT, AST;
- increased HDL levels in blood plasma;
- hyperphosphatemia (develops against the background of renal dysfunction).
Alfacalcidol, instructions for use (Method and dosage)
The drug is prescribed orally or intravenously.
Treatment begins with small doses, tests are performed once a week to determine the level of calcium and phosphorus in the blood.
Inside. The recommended daily dosage is 1 mcg. If well tolerated, it can be increased by 0.25-0.5 mcg per day. For children, dosage adjustment may be required depending on weight and age. Children over 3 years of age are prescribed from 0.01 to 0.08 mcg per kg of weight.
The duration of treatment depends on the disease, tolerability of the drug and the effectiveness of treatment. In some cases, the medicine is prescribed for life.
The drug is used intravenously at the end of a hemodialysis session in the form of a bolus. The injection is carried out into the return line of the device. A single dosage ranges from 1 to 6 mcg. The maximum amount of Alfacalcidol that can be administered to a patient during a week is 12 mcg.
Use of the drug Alfacalcidol
Orally, the duration of therapy is determined individually; if necessary, it is taken for life. For adults with vitamin D deficiency rickets and osteomalacia caused by exogenous vitamin D deficiency, diseases of the digestive system or long-term anticonvulsant therapy, a dose of 1–3 mcg/day is prescribed. For hypoparathyroidism, the daily dose is 2–4 mcg. For osteodystrophy in patients with chronic renal failure, the daily dose is 0.07-2 mcg, for Fanconi syndrome (hereditary renal acidosis with nephrocalcinosis, late rickets and adiposogenital dystrophy) and renal acidosis - 2-6 mcg. For hypophosphatemic rickets and osteomalacia - 4–20 mcg/day. For postmenopausal, senile, steroid and other types of osteoporosis, the daily dose is 0.5–1 mcg. It is recommended to start treatment with a minimum dose, monitoring the level of calcium and phosphorus in the blood plasma once a week. The dose of alfacalcidol can be increased by 0.25 or 0.5 mcg/day until biochemical parameters stabilize. Once the optimal effective dose is reached, it is recommended to monitor plasma calcium levels every 3–5 weeks. For children weighing less than 20 kg, alfacalcidol is prescribed at 0.01–0.05 mcg/kg per day; children weighing 20 kg or more - 1 mcg/day (except in cases of renal osteodystrophy). For renal osteodystrophy, the dose is 0.04–0.08 mcg/kg per day.
Overdose
When systematically taking large dosages of the drug, hypervitaminosis D .
Symptoms of hypercalcemia in the early stages: constipation or diarrhea , bone pain, drowsiness , muscle pain, metallic taste in the mouth, polydipsia , lack of appetite, fatigue, nocturia .
Symptoms of hypervitaminosis D in later stages: cloudy urine, painful sensations in the bones, leukocytonuria , elevated body temperature, conjunctivitis , ectopic calcification , increased blood pressure, decreased libido, photosensitivity , pancreatitis , rhinorrhea , psychosis , weight loss.
Signs of chronic vitamin D intoxication are: increased blood pressure, calcification of the kidneys , lungs, blood vessels, heart failure, even death, disruption of the normal growth process in young children.
As therapy, the medicine is discontinued. If an acute overdose occurred less than 2 hours ago, then you can rinse the stomach and give the victim mineral oil . Maintenance therapy, administration of infusion saline solutions , loop diuretics , bisphosphonates , glucocorticosteroids , and calcitonin . Hemodialysis is effective .
Alpha D3 Alfacalcidol caps 1 mcg x30
Trade name: Alpha D3-Teva International name: Alfacalcidol
Country of origin: Israel
Manufacturer: Teva Pharmaceutical Industries Ltd
Release forms: capsules 0.25, 1 mcg (polypropylene bottles)
Composition: alfacalcidol 0.25/1 mcg
Pharmacological group: vitamin - calcium-phosphorus metabolism regulator
Pharmacological group according to ATK: A11CC03 Alfacalcidol
Pharmacological action: D-vitamin, metabolic, antirachitic, regulating phosphorus-calcium metabolism
Drug registration number: P No. 012070/01
Dates of registration, re-registration: 12/29/2006
RF HS codes: 3004 50 100 9
Pharmacodynamics: A remedy that replenishes vitamin D3 deficiency. Regulator of phosphorus-calcium metabolism. A natural metabolite of 1 alpha, 25-dihydroxyvitamin D3 (calcitriol), the active form of vitamin D produced from vitamin D3 in the kidneys. Affects the nuclei of target cells and stimulates the transcription of DNA and RNA in the intestinal epithelium, bone tissue, renal parenchyma and skeletal muscles. Enhances the absorption of Ca2+ and phosphates in the intestine and their reabsorption in the proximal tubules of the kidneys, increases bone mineralization by stimulating the synthesis of osteocalcin in bone tissue, reduces the activity of alkaline phosphatase and the content of parathyroid hormone in the blood, normalizes the functions of muscle tissue, the growth and differentiation of cells of various types, increases cellular and humoral immunity. Restores a positive calcium balance in the treatment of calcium malabsorption syndrome, reduces the intensity of bone resorption and the incidence of fractures. During a course of treatment, it reduces bone and muscle pain associated with impaired phosphorus-calcium metabolism and improves coordination of movements. Duration of action - up to 48 hours.
Pharmacokinetics: Absorption - high, TCmax - 8-18 hours. In the blood it binds to specific alpha globulins. Metabolized in the liver to form the active metabolite calcitriol (1.25-dihydroxycolecalciferol), a smaller part is metabolized in bone tissue. Unlike natural vitamin D, it is not metabolized in the kidneys, which allows it to be prescribed for vitamin D deficiency in patients with renal failure (the effect does not depend on hydroxylation in the kidneys). T1/2 - 19 days. It is excreted by the kidneys and bile (in approximately the same ratio). Cumulates.
Indications: Hypo- and avitaminosis of vitamin D (prevention and treatment), as well as conditions of the body’s increased need for vitamin D: osteomalacia, osteoporosis, osteopathy (including after kidney transplantation, against the background of renal failure), poor and unbalanced nutrition ( including parenteral, vegetarian diet), malabsorption, insufficient insolation, hypocalcemia due to hypoparathyroidism, familial hypophosphatemia (vitamin D-resistant rickets), alcoholism, liver failure, liver cirrhosis, Fanconi syndrome (hereditary renal acidosis with nephrocalcinosis, late rickets and adiposogenital dystrophy), obstructive jaundice, gastrointestinal diseases (gluten enteropathy, persistent diarrhea, tropical sprue, Crohn's disease), rapid weight loss, pregnancy (especially with nicotine and drug addiction, multiple pregnancy), lactation period, breastfed newborns, with insufficient insolation, taking barbiturates, cholestyramine, colestipol, mineral oils, anticonvulsants (incl. phenytoin and primidone).
Dosage regimen: Inside. Adults: initial dose - 1 mcg / day, can be increased by 0.5 mcg every 2-4 weeks to 2 (in rare cases up to 3) mcg / day, maintenance dose - 0.25-1 mcg / day
children - 0.25 mcg/day.
Alpha D3-Teva: orally, for adults with osteomalacia caused by exogenous vitamin D deficiency, 1-3 mcg/day is prescribed, for osteodystrophy with chronic renal failure - 0.07-2 mcg, for Fanconi syndrome - 2-6 mcg, for hypophosphatemic rickets and osteomalacia - 4-20 mcg, for postmenopausal, senile, steroid osteoporosis - 0.5-1 mcg. Treatment is started with minimal doses, monitoring the content of Ca2+ and phosphorus in the plasma once a week; if necessary, the dose is increased by 0.25 or 0.5 mcg/day until biochemical parameters stabilize. Once the optimal effective dose is achieved, it is recommended to monitor plasma Ca2+ levels every 3-5 weeks.
Children weighing less than 20 kg are prescribed 0.01-0.05 mcg/kg/day, 20 kg and above - 1 mcg/day, for renal osteodystrophy - 0.04-0.08 mcg/kg/day.
Oksidevit: orally (capsules or 0.0009% solution in oil), regardless of food intake (1 drop = 0.25 mcg).
For children with rickets-like diseases - 0.5-3 mcg/day (2-12 drops), depending on age and body weight, for 2-3 months, if necessary - up to 1 year.
For rickets - 1 mcg/day daily for 10 days, 3 courses of treatment are carried out with a break of 2 weeks. For children on hemodialysis, in order to eliminate osteodystrophy - 1 mcg/day, with simultaneous use of vitamin D2 2000 IU or dioxivit - 20-40 mcg/day daily for a long time. Adults on hemodialysis, 1-2 mcg/day (4-8 drops) daily or every other day, followed by a dose reduction to 0.5 mcg, depending on the normalization of Ca2+ in the blood and alkaline phosphatase activity, treatment courses - 2-3 months , it is recommended to repeat 2-3 times a year.
After kidney transplantation for prophylactic purposes - 0.25-1 mcg/day daily or every other day. For bone pathologies of various origins - 0.5-3 mcg/day for a long time (from 2-3 to 12 months or more).
For osteomalacia associated with impaired absorption of Ca2+, phosphates or vitamin D in the intestine - 0.25-1.5 mcg/day.
Side effects: Allergic reactions.
Overdose.
Symptoms of vitamin D hypervitaminosis: early (due to hypercalcemia) - constipation or diarrhea, dry oral mucosa, headache, thirst, pollakiuria, nocturia, polyuria, anorexia, metallic taste in the mouth, nausea, vomiting, unusual fatigue, general weakness, hypercalcemia , hypercalciuria, late - bone pain, urine turbidity (appearance of hyaline casts in the urine, proteinuria, leukocyturia), increased blood pressure, skin itching, photosensitivity of the eyes, conjunctival hyperemia, arrhythmia, drowsiness, myalgia, nausea, vomiting, pancreatitis, gastralgia, weight loss , rarely - changes in psyche and mood (up to the development of psychosis).
Symptoms of chronic vitamin D intoxication: calcification of soft tissues, kidneys, lungs, blood vessels, arterial hypertension, renal and cardiovascular failure up to death (these effects most often occur when hyperphosphatemia is combined with hypercalcemia), impaired growth in children.
Treatment: discontinuation of the drug until the Ca2+ content in the plasma is normalized (usually for 1 week), then treatment can be resumed with half of the last dose used, in the early stages of acute overdose - gastric lavage, administration of mineral oil, which helps reduce absorption and increase excretion in feces . In severe cases - intravenous administration of 0.9% NaCl solution, loop diuretics, corticosteroids.
Contraindications: Hypersensitivity, hypercalcemia, hypervitaminosis D, renal osteodystrophy with hyperphosphatemia.
Interaction: Alfacalcidol increases the risk of developing heart rhythm disturbances due to cardiac glycosides. Ca2+ and phosphorus-containing drugs, as well as other drugs containing vitamin D, increase the risk of side effects (including hypercalcemia). Inducers of microsomal liver enzymes (for example, phenytoin and phenobarbital) reduce, and inhibitors increase, the concentration of alfacalcidol in plasma (possibly changing its effectiveness). Mineral oils, albumin-rich foods, cholestyramine, colistepol, sucralfate and antacids reduce the absorption of alfacalcidol. Taking antacids increases the risk of developing hypermagnesemia and hyperaluminemia; Ca2+ drugs and thiazide diuretics increase the risk of hypercalcemia. In the treatment of osteoporosis, it can be prescribed in combination with estrogens and other drugs that reduce bone resorption. The toxic effect is weakened by vitamin A, tocopherol, ascorbic acid, pantothenic acid, thiamine, riboflavin. Calcitonin, derivatives of etidronic and pamidronic acids, plicamycin, gallium nitrate and corticosteroids reduce the effect. Increases the absorption of phosphorus-containing drugs and the risk of hyperphosphatemia.
Special instructions: Therapy must be carried out under constant monitoring of the concentration of Ca2+ and phosphates in the blood (at the beginning of treatment - once a week, when Cmax is reached and throughout the entire period - the concentration of Ca2+ in plasma and urine every 3-5 weeks), as well as activity Alkaline phosphatase (for chronic renal failure - weekly monitoring). In case of chronic renal failure, preliminary correction of hyperphosphatemia is required. When normalizing the level of alkaline phosphatase in plasma, an appropriate dose reduction is necessary (to avoid the development of hypercalcemia). Hypercalcemia and hypercalciuria are corrected by discontinuing treatment and reducing Ca2+ intake (usually after 1 week). After normalization, therapy is continued, prescribing 1/2 of the last dose used. It should not be prescribed simultaneously with other vitamin D drugs and its derivatives. Use during pregnancy and lactation is possible if the expected benefit to the mother outweighs the potential risk to the fetus. It should be borne in mind that sensitivity to vitamin D varies from patient to patient, and in some patients taking even therapeutic doses can cause symptoms of hypervitaminosis. Newborns' sensitivity to vitamin D varies, and some may be sensitive to even very low doses. Children who receive vitamin D over a long period of time have an increased risk of stunted growth. To prevent hypovitaminosis D, a balanced diet is most preferable.
Breastfed newborns, especially those born to mothers with dark skin and/or insufficient sun exposure, are at high risk of developing vitamin D deficiency. Animal studies have shown that calcitriol, at doses 4 to 15 times higher than recommended human doses, has a teratogenic effect. Maternal hypercalcemia (associated with prolonged overdose of vitamin D during pregnancy) can cause increased sensitivity to vitamin D in the fetus, suppression of parathyroid function, specific elf-like appearance syndrome, mental retardation, and aortic stenosis. In old age, the need for vitamin D may increase due to a decrease in the absorption of vitamin D, a decrease in the skin's ability to synthesize provitamin D3, a decrease in sun exposure, and an increase in the incidence of renal failure.
Carefully. Atherosclerosis, sarcoidosis or other granulomatosis, CHF, history of nephrourolithiasis, hyperphosphatemia, chronic renal failure, pregnancy, lactation, childhood.
Category of action on the fetus. C
Storage conditions: List B
Interaction
Phenytoin , barbiturates , and antiepileptic drugs reduce the effectiveness of Alfacalcidol.
The combination of the drug with drugs that cause the induction of microsomal liver enzymes leads to a decrease in the effectiveness of the drug. It is recommended to increase the dosage of Alfacalcidol.
Concomitant use of the drug and digitalis preparations increases the likelihood of developing cardiac arrhythmia . When combined with calcium and calcium preparations , thiazide diuretics, there is a risk of hypercalcemia .
Antacids and laxatives increase the likelihood of developing hypermagnesemia .
When taking the drug in combination with mineral oils , colestipol , cholestyramine , antacids , albumin or sucralfate , its absorption capacity is reduced and the process of evacuation from the body is accelerated.
The medicine is not taken together with colecalciferol and its derivatives.
Carrying out laxative dialysis during treatment with Alfacalcidol increases the likelihood of developing hypermagnesemia and hyperaluminemia .
Drug interactions Alfacalcidol
When used simultaneously with alfacalcidol and digitalis preparations, the risk of developing heart rhythm disturbances increases. When co-administered with barbiturates, anticonvulsants and other drugs that activate microsomal liver enzymes, it is necessary to prescribe alfacalcidol at a higher dose. Absorption of alfacalcidol decreases when used simultaneously with mineral oils, cholestyramine, colestipol, sucralfate, antacids, and albumin-based drugs. With simultaneous use of antacids, the risk of developing hypermagnesemia increases. Concomitant use of calcium supplements and thiazide diuretics increases the risk of developing hypercalcemia. Vitamin D and its derivatives should not be administered during alfacalcidol therapy due to possible additive interactions and an increased risk of hypercalcemia.
special instructions
During treatment with the drug, it is recommended to periodically monitor the level of calcium and phosphate in the blood. At the initial stages of treatment, tests are carried out every 3-4 days, then after stabilization of the patient’s condition - every 3-5 weeks. Once every 2 months it is recommended to determine the level of calcium in the urine and the level of alkaline phosphatase.
If chronic renal failure was detected in the patient before starting the drug, it is necessary to correct hyperphosphatemia . When the level of alkaline phosphatase in the blood plasma has returned to normal, it is recommended to reduce the dosage.
Intravenous administration of the drug to newborn premature infants is prescribed with extreme caution.
Hypercalcemia occurs with kidney disease, high dosages of medication and an increased degree of demineralization . Therefore, after normalization of the level of alkaline phosphatase in the blood serum or when biochemical signs of normalization of bone structure appear, it is necessary to reduce the dosage of Alfacalcidol.
In patients with renal osteodystrophy, phosphate binders to prevent the development of hyperphosphatemia .
If during treatment the patient develops hypercalciuria , then therapy is stopped and after some time Alfacalcidol is prescribed in smaller doses. The degree of sensitivity to vitamin D is strictly individual.
To prevent hypovitaminosis, it is recommended to eat a balanced diet.
Unlike natural vitamin D , this drug does not undergo metabolic reactions in the kidneys, which makes it possible to prescribe the drug to patients with renal failure.
There are reports that the drug has proven to be quite effective during the treatment of secondary hyperparathyroidism resulting from renal failure.
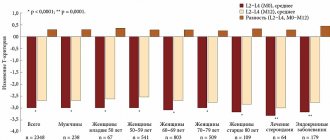
The successes of modern medicine have led to an increase in people's life expectancy, but at the same time to an increase in the prevalence of some “age-related” diseases. These include osteoporosis (OP), a systemic skeletal disease accompanied by bone fragility. Elderly people and seriously ill patients with secondary AP experience a fear of falls, as this can lead to fractures. This fear is justified, because... low-energy fractures, especially of the proximal femur, lead to hospitalization, loss of functionality, the need for outside care, and are one of the causes of death.
Of course, doctors currently have a large selection of modern medicines in their arsenal that can improve the quantitative and qualitative characteristics of bone tissue and reduce the risk of developing fractures. In modern clinical practice, correction of vitamin D deficiency plays a significant role.
Vitamin D, which comes from food and dietary supplements, and is also produced in the skin from sun exposure, is biologically inert. To convert it into an active metabolite, which subsequently binds to specific receptors in tissues, two successive hydroxylation reactions are necessary: first, in the liver, native vitamin D under the influence of 25-hydroxylase is converted into 25-hydroxyvitamin D (25(OH)D), or calcidiol. Calcidiol is then further hydroxylated in various tissues (mainly the kidneys) by the enzyme 1α-hydroxylase to form physiologically active 1α,25-dihydroxyvitamin D (1α,25(OH)2D), known as D-hormone, or calcitriol [1]. The molecular mechanism of action of the D-hormone is similar to other steroid hormones and consists of interaction in tissues with specific receptors called vitamin D receptors (VDR), widely represented not only in the classical target organs for vitamin D (intestines, kidneys and bones), but also in other organs and tissues [2].
The pathophysiological mechanisms of the development of AP are associated not only with vitamin D deficiency/deficiency, but also with impaired D-hormone synthesis, a decrease in the number of VDRs and/or loss of their sensitivity in target organs. As a result, the activity of bone cells changes, the processes of remodeling and mineralization of bone tissue are disrupted. A decrease in D-hormone production and VDR affinity leads to disruption of the normal functioning of the neuromuscular system. In older people, in parallel with a decrease in bone strength, there is a loss of muscle mass and strength (sarcopenia), gait and posture change, and the risk of falls increases. The type of falls also changes; usually older people fall on their side rather than straight, which, along with loss of fat mass, leads to hip fractures.
A decrease in D-hormone synthesis is observed in both primary and secondary AP. The most pronounced changes as a result of inhibition of 1α-hydroxylase in the kidneys occur in the elderly, patients with reduced renal function, nephropathies, hypertensive nephroangiosclerosis, in patients with chronic inflammatory diseases (rheumatoid arthritis, Crohn's disease, chronic obstructive pulmonary disease), type 1 diabetes mellitus, atherosclerosis and heart failure. In these cases, resistance to therapy with native vitamin D is possible. A similar situation can be observed in patients with the absence of receptors or a decrease in their affinity for D-hormone (VDR deficiency) in target organs (for example, in the gastrointestinal tract, bones, muscles), which is often observed in old age or during therapy with glucocorticosteroids. It has been shown that a decrease in creatinine clearance <65 ml/min in individuals over 70 years of age leads to a significant drop in serum D-hormone levels and a 4-fold increase in the risk of fractures compared to individuals with normal creatinine clearance [3].
Based on natural vitamins D2 and D3, their active metabolites and synthetic analogues, a significant number of vitamin and drug preparations have been created in the form of various dosage forms, which are used for the prevention and treatment of not only AP, but also other diseases. Vitamin D preparations are divided into two groups: variants of native vitamin D (D2 - ergocalciferol, D3 - cholecalciferol) and active metabolites of vitamin D3 (calcitriol, alfacalcidol).
Preparations of native vitamin D undergo 25-hydroxylation in the liver, followed by conversion in the kidneys into active metabolites. Their metabolic processes may be weakened in patients with primary and secondary AP, especially with concomitant liver and kidney diseases, as well as when taking certain medications. Active metabolites do not have these disadvantages. In addition, unlike native vitamin D, which is effective in reducing 25(OH)D below 30 nmol/L, active metabolites can increase D-hormone levels in target organs (rather than serum) in the absence of vitamin D deficiency [3]. . The drug with the non-proprietary international name "calcitriol" is chemically identical to D-hormone. Another drug in this group (alfacalcidol) is a synthetic 1α-derivative of the active metabolite of vitamin D (1α(OH)D3).
Alfacalcidol is a prodrug, i.e. a substance that is activated in the liver and bone tissue to form D-hormone after administration into the body. Unlike native vitamin D, alfacalcidol does not require hydroxylation in the kidneys, which allows its use in patients with reduced function and kidney disease. The mechanisms of action of calcitriol and alfacalcidol are similar to those of natural D-hormone, but differ in pharmacokinetic parameters, tolerability and some features of pharmacological action [2]. The action of calcitriol develops faster than alfacalcidol and is accompanied by a more pronounced hypercalcemic effect. To maintain a stable therapeutic concentration, it is necessary to prescribe calcitriol at least 2-3 times a day, while the effect of alfacalcidol develops more slowly but lasts longer after a single dose [2].
Given the characteristics of active D metabolites, their effect should not be monitored by testing 25(OH)D in serum. The combination of active metabolites with native vitamin D preparations and combination preparations of native vitamin D and calcium is not recommended.
Active metabolites of vitamin D enhance the active absorption of calcium and phosphates, affect bone remodeling processes and normalize the function of the neuromuscular system. They reduce the level of parathyroid hormone, the release of pro-inflammatory cytokines, which are a factor in the activation of osteoclasts and the acceleration of bone resorption and muscle weakness. Studies have shown that alfacalcidol not only inhibits osteoclastogenesis due to a decrease in the pool of osteoclast precursors in the bone marrow, but also promotes the process of bone formation and thus links both processes of bone remodeling [3]. These properties of the drug allow it to be used by patients with low bone turnover. Alfacalcidol is more effective than native vitamin D in increasing bone mineral density (BMD) and bone strength, independent of calcium absorption and parathyroid hormone suppression.
According to the results of prospective randomized placebo-controlled trials [4–7] and a prospective epidemiological cohort study [8], active vitamin D metabolites increase BMD and reduce the incidence of vertebral and non-vertebral fractures. Several meta-analyses have been conducted indicating the effectiveness of active metabolites of vitamin D (alfacalcidol and calcitriol) and their advantage over native vitamin D in preventing loss of BMD and the development of vertebral fractures in primary osteoporosis [9–11].
According to studies, the combination of alfacalcidol with alendronate [12] or denosumab [13] leads to a more significant increase in BMD than the combination of these drugs with native vitamin D.
The use of vitamin D supplements has proven to be the most effective intervention in attempts to reduce the incidence of falls in patients with osteoporosis through drug interventions. They normalize the calcium/phosphorus ratio, improving muscle contractility and plasticity; influence the differentiation of muscle fibers, the expression of contractile proteins, mitochondrial metabolism; increase the number of muscle fibers and the number of neuromuscular synapses [1]. The effect of native vitamin D in this regard is weaker compared to the active metabolites of D. In a small uncontrolled study examining biopsy material obtained from elderly women after treatment with alfacalcidol and calcium supplements for 3-6 months, an increase in the number and volume of IIa muscle fibers was shown type [14]. After 6 months of alfacalcidol therapy in a group of elderly women with D-hormone deficiency, an increase in muscle strength (as measured by isometric knee stretching) and improvement in functional indicators (walking for 2 minutes) were noted [15]. Alfacalcidol has been shown to significantly reduce the number of falls in elderly people living in nursing homes [16]. A meta-analysis combining 14 studies (21,268 patients) showed that active metabolites of vitamin D more significantly reduced the risk of falls (by 21%) compared with colecalciferol (by 6%) [17].
The use of active metabolites of vitamin D is pathogenetically justified for the treatment of glucocorticoid AP. These drugs have been shown to be more effective in preventing BMD loss and reducing the risk of vertebral fractures compared with native vitamin D, calcium monotherapy, placebo, or no treatment. Analysis of the effectiveness of active metabolites of vitamin D in separate subgroups showed that the use of alfacatacidol prevented vertebral fractures, and calcitriol showed only a tendency towards a protective effect [18]. Active metabolites of vitamin D as monotherapy are included in European [19] and national clinical guidelines for the management of patients with glucocorticoid AP [20].
Active metabolites have a favorable safety profile. The most common side effects of these drugs are hypercalcemia and hyperphosphatemia, which is associated with one of the main mechanisms of their action - increased intestinal absorption of calcium and phosphorus. Therefore, during treatment, the general condition of the patient, as well as the levels of calcium and phosphorus in the blood, should be monitored. If hypercalcemia occurs, treatment should be discontinued for 7–10 days and then restarted using half the dose [2].
Thus, preparations of active metabolites of vitamin D have a therapeutic effect in various forms of AP and can be used both as monotherapy and in combination with other anti-osteoporotic drugs.
Drugs containing (Alfacalcidol analogues)
Level 4 ATX code matches:
Etalfa
Dihydrotachysterol
Oksidevit
Tevabon
Alpha D3-Teva
Ergocalciferol
Aquadetrim
Vigantol
Synonyms: Alfadol , Oxidevit , Etfa , Alpha D3-Teva , Van-Alfa , Oxidevit capsules .
In combination with calcium carbonate, the substance is found in the drug Alfadol-Ca . The product is also included in Tevabon in combination with alendronic acid .