Linezolid Canon, 600 mg, film-coated tablets, 10 pcs.
In an open-label study of critically ill patients with intravascular catheter-associated infections, there was an excess of mortality in patients receiving linezolid compared with patients receiving vancomycin/dicloxacillin/oxacillin [78/363 (21.5%) vs 58/363 (16.5%). 0%)]. The main factor influencing mortality was the gram-positive pathogen at the initial stage. The mortality rate was similar among patients whose infections were caused only by gram-positive organisms, but was significantly higher in the linezolid group when other organisms were also detected or were not initially detected. The greatest imbalance was noted during treatment and within 7 days after the end of antibiotic therapy. Many patients in the linezolid group developed Gram-negative organisms during the study and died from Gram-negative or polymicrobial infections. Therefore, for complicated skin and soft tissue infections, linezolid should be used in patients with known or possible co-infection with Gram-negative organisms only if no alternative treatment options are available. In these cases, additional use of drugs acting on gram-negative microflora is simultaneously indicated.
Some patients taking linezolid may develop reversible myelosuppression (with anemia, thrombocytopenia, leukopenia and pancytopenia), depending on the duration of therapy. Older patients are also at increased risk of developing this condition. Thrombocytopenia occurred more often in patients with severe renal failure, regardless of the patient's use of hemodialysis. In this regard, during treatment it is necessary to monitor blood counts in patients with an increased risk of bleeding, a history of myelosuppression, as well as with simultaneous use of drugs that reduce hemoglobin or platelet count and/or their functional properties, with severe renal failure, as well as in patients taking linezolid for more than 2 weeks. Linezolid is used in such patients only when close monitoring of hemoglobin, white blood cell and platelet counts is possible. If significant myelosuppression develops during linezolid therapy, treatment should be discontinued unless continued therapy is considered absolutely necessary. In this case, intensive monitoring of blood counts and appropriate treatment are necessary. In addition, it is recommended that blood tests (including hemoglobin, platelet count, and white blood cell count (with leukocyte count calculation)) be performed weekly in patients receiving linezolid, regardless of baseline blood test values. A higher incidence of severe anemia was observed in patients receiving linezolid for more than the maximum recommended duration of 28 days. These patients were more likely to require blood transfusions. Cases of sideroblastic anemia have been reported in the post-marketing period. In most cases, the duration of linezolid therapy exceeded 28 days. In most patients, manifestations were completely or partially reversible after discontinuation of linezolid treatment with or without specific anemia treatment.
In patients taking antibacterial drugs, including linezolid, the risk of developing pseudomembranous colitis of varying severity should be considered. About cases of diarrhea associated with Clostridium difficile
, has been reported in association with the use of virtually all antibacterial drugs, including linezolid.
The severity of diarrhea can vary from mild to severe. Treatment with antibacterial drugs disrupts the normal intestinal microflora, which leads to overgrowth of Clostridium difficile
.
Clostridium difficile
Clostridium difficile-
associated diarrhea .
Excessive amounts of toxins produced by Clostridium difficile
may cause increased mortality in patients, as such infections may be resistant to antimicrobial therapy and may require colonectomy.
Do not use medications that inhibit intestinal motility. Clostridium difficile -associated diarrhea
should be considered in all patients with diarrhea following antibiotic use.
Close medical observation for 2 months is necessary for patients who experience diarrhea associated with Clostridium difficile
after administration of antibacterial drugs.
If symptoms of deterioration in visual function appear, such as changes in visual acuity, changes in color perception, blurred vision, visual field defects, it is recommended to immediately consult an ophthalmologist for consultation. Visual function should be monitored in all patients taking linezolid long-term (more than 28 days) and in all patients with new-onset visual symptoms, regardless of the duration of therapy.
In the event of development of peripheral neuropathy and optic neuropathy, the risk/benefit ratio of continuing linezolid therapy in these patients should be assessed. The risk of developing neuropathy is higher if linezolid is used in patients who are currently using or who have recently taken antibacterial drugs to treat tuberculosis.
Lactic acidosis has been reported in association with linezolid use. Patients who experience repeated nausea or vomiting, abdominal pain, unexplained acidosis, or a decrease in bicarbonate anion concentrations while taking linezolid require careful monitoring by a physician.
Linezolid inhibits mitochondrial protein synthesis. Side effects such as lactic acidosis, anemia and neuropathy (peripheral and optic) may occur as a result of this inhibition; these effects are more common when the drug is used for more than 28 days.
Convulsions have been reported in patients taking linezolid, with most cases having a history of convulsions or risk factors for their development. Patients should obtain a detailed history regarding previous episodes of seizures.
If it is necessary to use the drug in combination with selective serotonin reuptake inhibitors, patients should be constantly monitored to identify signs and symptoms of serotonin syndrome, such as impaired cognitive function, hyperpyrexia, hyperreflexia and impaired motor coordination. If these symptoms appear, one or both medications should be discontinued. When you stop taking a serotonergic drug, withdrawal symptoms may occur.
Cases of reversible superficial discoloration of tooth enamel have been reported with the use of linezolid. These discolorations were removed by professional teeth cleaning.
Cases of symptomatic hypoglycemia have been reported in patients with diabetes mellitus receiving linezolid concomitantly with insulin or hypoglycemic agents. Although a cause-and-effect relationship between the use of linezolid and the development of hypoglycemia has not been established, patients with diabetes mellitus should be warned about the possibility of developing hypoglycemia. If hypoglycemia occurs, dose adjustment of insulin/hypoglycemic drugs or discontinuation of linezolid is necessary.
Patients should be advised not to consume large quantities of foods containing tyramine (such as red wine, old cheese, some alcoholic beverages, smoked meats).
There have been no clinical studies examining the effect of linezolid on the normal microflora of the human body.
The use of antibacterial drugs can sometimes lead to increased growth of microorganisms that are resistant to it. Clinical studies have shown that approximately 3% of patients receiving recommended doses of linezolid developed antibiotic-associated candidiasis. If superinfection occurs while taking linezolid, appropriate medical measures should be taken.
Clinical researches.
The safety and effectiveness of linezolid for more than 28 days have not been established.
Controlled clinical trials did not include patients with diabetic foot syndrome, pressure ulcers or ischemic disorders, severe burns or gangrenous lesions. Thus, experience with linezolid in the treatment of these conditions is limited.
Impact on the ability to drive vehicles and machinery
During the treatment period, driving and engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions are not recommended.
Linezolid (Linezolidum)
In an open-label study of critically ill patients with intravascular catheter-associated infections, there was an excess of mortality in patients receiving linezolid compared with patients receiving vancomycin/dicloxacillin/oxacillin [78/363 (21.5%) vs 58/363 (16.0%). %)].
The main factor influencing mortality was the gram-positive pathogen at the initial stage. The mortality rate was similar among patients whose infections were caused only by gram-positive organisms, but was significantly higher in the linezolid group when other organisms were also detected or were not initially detected. The greatest imbalance was noted during treatment and within 7 days after the end of antibiotic therapy. During the study, more patients in the linezolid group acquired gram-negative organisms and subsequently died from gram-negative or polymicrobial infections. Therefore, for complicated skin and soft tissue infections, linezolid should be used in patients with known or possible co-infection with Gram-negative organisms only if no alternative treatment options are available. In these cases, additional use of drugs acting on gram-negative microflora is simultaneously indicated.
In some patients taking linezolid. Reversible myelosuppression (with anemia, thrombocytopenia, leukopenia and pancytopenia) may develop, depending on the duration of therapy. Older patients are also at increased risk of developing this condition. Thrombocytopenia occurred more often in patients with severe renal failure, regardless of the patient's use of hemodialysis. In this regard, during treatment it is necessary to monitor blood counts in patients with an increased risk of bleeding, a history of myelosuppression, as well as with simultaneous use of drugs that reduce hemoglobin or platelet count and/or their functional properties, with severe renal failure, as well as in patients receiving linezolid for more than 2 weeks. Linezolid is used in such patients only when close monitoring of hemoglobin, white blood cell and platelet counts is possible. If significant myelosuppression develops during linezolid therapy, treatment should be discontinued unless continued therapy is considered absolutely necessary. In this case, intensive monitoring of blood counts and appropriate treatment are necessary. In addition, it is recommended that blood tests (including hemoglobin, platelet count, and white blood cell count (with leukocyte count calculation)) be performed weekly in patients receiving linezolid, regardless of baseline blood test values. A higher incidence of severe anemia was observed in patients receiving linezolid for more than the maximum recommended duration of 28 days. These patients required blood transfusions. Cases of sideroblastic anemia have been reported in the post-marketing period. In most cases, the duration of linezolid therapy exceeded 28 days. In most patients, manifestations were completely or partially reversible after discontinuation of linezolid treatment with or without specific anemia treatment. In patients taking antibacterial drugs, including linezolid, the risk of developing pseudomembranous colitis of varying severity should be considered.
About cases of diarrhea associated with Clostridium difficile,
has been reported in association with the use of virtually all antibacterial drugs, including linezolid.
The severity of diarrhea can range from mild to severe. Treatment with antibacterial drugs disrupts the normal intestinal flora, leading to overgrowth of Clostridium difficile.
Clostridium difficile produces toxins A and B, which lead to Clostridium difficile-associated diarrhea
.
Excessive amounts of toxins produced by
Clostridium difficile
may cause increased mortality in patients, as such infections may be resistant to antimicrobial therapy and may require colonectomy. Do not use medications that inhibit intestinal motility.
Clostridium difficile- associated diarrhea
should be considered in all patients with diarrhea following antibiotic use.
Close medical observation for 2 months is necessary for patients who experience diarrhea associated with Clostridium difficile
after administration of antibacterial drugs. If symptoms of deterioration in visual function appear, such as changes in visual acuity, changes in color perception, blurred vision, visual field defects, it is recommended to immediately consult an ophthalmologist for consultation. Visual function should be monitored in all patients taking linezolid long-term (more than 28 days) and in all patients with new-onset symptoms of visual impairment, regardless of the duration of therapy. In the event of development of peripheral neuropathy and optic neuropathy, the risk/benefit ratio of continuing linezolid therapy in these patients should be assessed. The risk of developing neuropathy is higher if linezolid is used in patients who are currently using or who have recently taken antimycobacterial drugs to treat tuberculosis.
Lactic acidosis has been reported in association with linezolid use. Patients who experience repeated nausea or vomiting, abdominal pain, unexplained acidosis, or a decrease in bicarbonate anion concentrations while taking linezolid require careful monitoring by a physician. Linezolid inhibits mitochondrial protein synthesis. Side effects such as lactic acidosis, anemia, and neuropathy (peripheral or optic) may result from this inhibition; these effects are more common when the drug is used for more than 28 days.
Convulsions have been reported in patients taking linezolid, with most cases having a history of convulsions or risk factors for their development. Patients should obtain a detailed history regarding previous episodes of seizures.
If it is necessary to use the drug in combination with selective serotonin reuptake inhibitors, patients should be constantly monitored to identify signs and symptoms of serotonin syndrome, such as impaired cognitive function, hyperpyrexia, hyperreflexia and impaired motor coordination. If these symptoms appear, one or both medications should be discontinued. When you stop taking a serotonergic drug, withdrawal symptoms may occur.
Cases of reversible superficial discoloration of tooth enamel have been reported with the use of linezolid. These discolorations were removed by professional teeth cleaning.
Cases of symptomatic hypoglycemia have been reported in patients with diabetes mellitus receiving linezolid concomitantly with insulin or hypoglycemic agents. Although a cause-and-effect relationship between the use of linezolid and the development of hypoglycemia has not been established, patients with diabetes mellitus should be warned about the possibility of developing hypoglycemia. If hypoglycemia occurs, dose adjustment of insulin/hypoglycemic drugs or discontinuation of linezolid is necessary. Patients should be advised not to consume large quantities of foods containing tyramine (such as red wine, old cheese, some alcoholic beverages, smoked meats).
There have been no clinical studies examining the effect of linezolid on the normal microflora of the human body. The use of antibacterial drugs can sometimes lead to increased growth of microorganisms that are resistant to it. Clinical studies have shown that approximately 3% of patients receiving recommended doses of linezolid developed antibiotic-associated candidiasis. If superinfection occurs while taking linezolid, appropriate medical measures should be taken. Clinical researches
The safety and effectiveness of linezolid for more than 28 days have not been established. Controlled clinical trials did not include patients with diabetic foot syndrome, pressure ulcers or ischemic disorders, severe burns or gangrenous lesions. Thus, experience with linezolid in the treatment of these conditions is limited.
Linezolid-teva 2mg/ml 300ml 10 pcs. solution for infusion
pharmachologic effect
Antimicrobial agent, belongs to the class of oxazolidinones.
The mechanism of action is due to the selective inhibition of protein synthesis in bacteria. By binding to bacterial ribosomes, linezolid prevents the formation of a functional 70S initiation complex, which is a component of the translation process during protein synthesis. Active against aerobic gram-positive bacteria: Corynebacterium jeikeium, Enterococcus faecalis (including glycopeptide-resistant strains), Enterococcus faecium (including glycopeptide-resistant strains), Enterococcus casseliflavus, Enterococcus gallinarum, Listeria monocytogenes, Staphylococcus aureus (including methicillin-resistant strains), Staphylococcus aureus (strains with intermediate sensitivity to glycopeptides), Staphylococcus epidermidis (including methicillin-resistant strains), Staphylococcus haemolyticus, Staphylococcus lugdunensis, Streptococcus agalactiae, Streptococcus intermedius, Streptococcus pneumoniae (including strains with intermediate sensitivity to penicillin and penicillin-resistant strains), Str eptococcus spp. (streptococci of groups C and G), Streptococcus pyogenes, Streptococcus viridans; aerobic gram-negative bacteria: Pasteurella canis, Pasteurella multocida; anaerobic gram-positive bacteria: Clostridium perfringens, Peptostreptococcus spp. (including Peptostreptococcus anaerobius); anaerobic gram-negative bacteria: Bacteroides fragilis, Prevotella spp.; Chlamydia pneumoniae.
Less active against Legionella spp., Moraxella catarrhalis, Mycoplasma spp.
Not active against Haemophilus influenzae, Neisseria spp., Enterobacteriaceae, Pseudomonas spp.
There was no cross-resistance between linezolid and aminoglycosides, beta-lactam antibiotics, folic acid antagonists, glycopeptides, lincosamides, quinolones, rifamycins, streptogramins, tetracyclines, chloramphenicol. The mechanism of action of linezolid differs from the mechanisms of action of these antibacterial drugs.
Resistance to linezolid develops slowly through a multistep mutation of 23S ribosomal RNA and occurs at a frequency of less than 1x10-9-1x10-11.
Composition and release form Linezolid-teva 2 mg/ml 300 ml 10 pcs. solution for infusion
Solution - 1 ml: linezolid 2 mg.
300 ml - infusion bags (10) - cardboard boxes.
Directions for use and doses
The dosage regimen and duration of treatment depend on the causative agent, the location and severity of the infection, as well as the clinical effectiveness.
Administered intravenously in the form of infusions at a dose of 600 mg with an interval of 12 hours. Duration of treatment is 14-28 days.
Patients who were prescribed the drug intravenously at the beginning of therapy can subsequently be switched to any dosage form for oral administration. In this case, dose selection is not required, because bioavailability when taken orally is almost 100%.
Pharmacokinetics
Linezolid is rapidly distributed into well-perfused tissues. Vd upon reaching Css in healthy volunteers averages 40-50 l. Plasma protein binding is 31% and is independent of the concentration of linezolid in the blood.
It has been established that cytochrome P450 isoenzymes are not involved in the metabolism of linezolid in vitro. Linezolid also does not inhibit the activity of clinically important cytochrome P450 isoenzymes (1A2, 2C9, 2C19, 2D6, 2E1, 3A4). Metabolic oxidation leads to the formation of 2 inactive metabolites - hydroxyethylglycine (which is the main metabolite in humans and is formed as a result of a non-enzymatic process) and aminoethoxyacetic acid (formed in smaller quantities). Other inactive metabolites have also been described.
Linezolid is excreted mainly in the urine as hydroxyethylglycine (40%), aminoethoxyacetic acid (10%) and unchanged drug (30-35%). Excreted in feces in the form of hydroxyethylglycine (6%) and aminoethoxyacetic acid (3%). The unchanged drug is practically not excreted in the feces.
Indications for use Linezolid-teva 2 mg/ml 300 ml 10 pcs. solution for infusion
Treatment of infectious and inflammatory diseases caused by sensitive to anaerobic and aerobic gram-positive microorganisms (including infections accompanied by bacteremia): community-acquired pneumonia; hospital pneumonia; skin and soft tissue infections; infections caused by Enterococcus spp. (including strains of Enterococcus faecalis and Enterococcus faecium resistant to vancomycin).
Infections caused by gram-negative microorganisms, confirmed or suspected (as part of combination therapy).
Contraindications
Hypersensitivity to linezolid.
Application of Linezolid-teva 2 mg/ml 300 ml 10 pcs. solution for infusion during pregnancy and lactation
Adequate and strictly controlled studies of the safety of linezolid during pregnancy have not been conducted. The use of linezolid during pregnancy is possible only in cases where the expected benefit of therapy for the mother outweighs the potential risk to the fetus
It is not known whether linezolid is excreted in breast milk, therefore, if it is necessary to use the drug during lactation, breastfeeding should be discontinued.
special instructions
If diarrhea develops while using linezolid, the risk of developing pseudomembranous colitis of varying severity should be taken into account.
During treatment, it is necessary to conduct a clinical blood test in patients with an increased risk of bleeding, a history of myelosuppression, as well as in concomitant use of drugs that reduce hemoglobin levels, platelet counts or their functional properties, as well as in patients receiving linezolid for more than 2 weeks.
Side effects Linezolid-teva 2mg/ml 300ml 10 pcs. solution for infusion
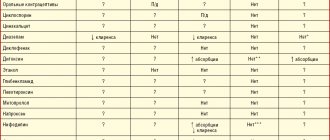
From the digestive system: often (>1%) - taste perversion, nausea, vomiting, diarrhea, abdominal pain (including cramping), flatulence, changes in total bilirubin, ALT, AST, alkaline phosphatase.
From the hematopoietic system: often (>1%) - reversible anemia, thrombocytopenia, leukopenia, pancytopenia.
Other: often (>1%) - headache, candidiasis; rarely - cases of peripheral neuropathy and optic neuropathy when used for more than 28 days (the relationship between the use of linezolid and the development of neuropathy has not been proven).
Adverse reactions are not dose dependent and, as a rule, do not require discontinuation of treatment.
Drug interactions
Linezolid is a weak, reversible, non-selective MAO inhibitor; therefore, in some cases, linezolid can cause a moderate, reversible increase in the pressor effect of pseudoephedrine and phenylpropanolamine. Taking this into account, when used simultaneously, it is recommended to reduce the initial doses of adrenergic drugs (including dopamine and its agonists) and subsequently titrate the dose.