Idebenone is a synthetic analogue of Coenzyme Q10. Coenzyme Q10 (ubiquinone) is a vitamin-like compound that is produced in the liver provided that vitamins B2, B3, B6, C, folic and pantothenic acids are supplied in the required amount. If there is a deficiency of any of these vitamins, the synthesis of coenzyme Q10 is inhibited. Ubiquinone stimulates the production of the main energy molecule - adenosine triphosphate (ATP). With age, the content of coenzyme in the body decreases, and it is advisable to use it to slow down the aging process and prevent degenerative diseases [8].
The use of natural coenzyme Q10 has a number of limitations. The ubiquinone molecule is large (50 carbon residues in the side chain), and the presence of high hydrophobicity makes it difficult to pass through membranes. In addition, under some conditions, instead of an antioxidant effect, the prooxidant properties of the compound may appear [5, 6]. In order to overcome these shortcomings, attempts have been made to synthesize derivatives of coenzyme Q10 that retain all of its positive properties of its natural analogue, but are devoid of its disadvantages. To date, only one of the many synthesized molecules of this series has received approval as a drug.
Such a synthetic derivative of ubiquinone is idebenone (trade name - noben), which, compared to coenzyme Q10, has a 5 times smaller size, less hydrophobicity and greater antioxidant activity [2]. Idebenone - (gross formula C19H30O5) is a benzoquinone derivative, has a molecular weight of 338.4 amu, penetrates well through the blood-brain barrier and is distributed in significant quantities into brain tissue. The drug also discovered some new properties. Thus, in animal experiments it was found that with long-term use, idebenone enhances protein-nucleic acid synthesis in the brain, stimulates the synthesis of nerve growth factor (NGF) and exhibits serotonergic activity [7, 32].
Idebenone slows down lipid peroxidation, protecting the membranes of neurons and mitochondria from damage. Coenzyme Q10 is one of the components of the electron transport chain located within the inner mitochondrial membrane. It accepts electrons from complexes I (NADH-ubiquinone oxidoreductase) and II (succinate dehydrogenase) for subsequent transfer to complex III (ubiquinone-cytochrome c reductase). Oxidative phosphorylation, realized through the production of ATP, is an essential process used in the process of energy production to maintain the functions of organs such as the brain, heart, and muscles [10]. Along with metabolic and trophic effects, idebenone also has nootropic properties. By increasing metabolic processes in the brain by activating the synthesis of glucose and ATP, improving blood supply and tissue nutrition, it also promotes the elimination (removal) of lactates.
In clinical practice, idebenone increases the speed of sensorimotor reactions, has a pronounced specific effect on cognitive processes, improving short-term and working memory, as well as attention [23]. In addition, the compound has a pronounced antioxidant effect. Thus, the indications for prescribing the drug are: cerebrovascular disorders, asthenic conditions, psychoorganic syndrome, emotionally labile disorders. From the first days of taking idebenone, it exhibits antiasthenic, psychostimulating and antidepressant effects; the nootropic effect appears after 3-4 weeks of use [13]. The overall cytoprotective effectiveness of the drug determines its use in gerontology. Considering the fact that mitochondrial deficiency is detected in many hereditary degenerative diseases of the central nervous system, the possibilities of using idebenone in these diseases are being actively studied [21, 34]. Idebenone has been shown to be effective in the complex therapy of hereditary mitochondrial diseases (MELAS syndrome, Leber optic atrophy, Leigh's disease) and Friedreich's ataxia.
After oral administration, idebenone is absorbed quite quickly and completely from the gastrointestinal tract, but its bioavailability through this route of administration is very low due to first-pass metabolism (“first-pass effect”). Concomitant food intake affects the bioavailability of the drug, while the concentration of native idebenone in plasma increases approximately 5 times, and therefore it is recommended to take the drug orally after meals. The pharmacokinetics of native idebenone and its metabolites is linear after both single and repeated use [22]. In the body, idebenone is metabolized by oxidation and sequential shortening of the side chain with the formation of pharmacologically inactive metabolites: QS10, QS8, QS6 and QS4. Native idebenone and its metabolites are present in biological fluids either in free or conjugated (glucuronoconjugates and sulfonic conjugates) form. The drug is excreted primarily by the kidneys, and to a lesser extent through the gastrointestinal tract. Idebenone does not accumulate (including active metabolites) even with long-term use [27].
Idebenone (Noben) is prescribed orally, after meals, 2-3 times a day. The results of clinical studies demonstrate good tolerability of idebenone in a daily dose of up to 3000 mg [12]. “Mild” adverse events such as headache, heartburn and other gastrointestinal disorders (dyspepsia) are rarely observed. Other adverse reactions include asthenia, involuntary movements, asymptomatic increases in liver enzymes and allergic reactions.
A contraindication to taking idebenone is chronic renal failure. The possibility of drug interactions with idebenone should be taken into account in patients suffering from concomitant somatic diseases: the drug has a positive inotropic effect and helps lower blood pressure; has synergism with blood sugar-lowering agents (risk of hypoglycemia). Idebenone in vitro
is capable of inhibiting platelet aggregation, which should be taken into account when prescribing anticoagulants simultaneously and indicating a history of hemorrhagic complications.
Use of idebenone (Noben) in clinical practice
Currently, there is convincing evidence of the role of oxidative stress and defects in energy metabolism in the pathogenesis of many neurodegenerative disorders, such as Friedreich's ataxia, Parkinson's disease, Huntington's chorea and Alzheimer's disease [9, 17, 19]. Thus, the rationale for testing idebenone as a potential neuroprotective agent is obtained [5, 31]. Coenzyme Q10 levels in the brain and other tissues of humans and animals decline with age. In addition, the lowest level of coenzyme Q10 was found in the substantia nigra, the cell death of which leads to the development of symptoms of Parkinson’s disease [24]. In recent years, a number of clinical studies have been conducted [10, 11, 15, 20, 25, 28] to study the effectiveness of coenzyme Q10 in neurodegenerative diseases.
T. Meier and G. Buyse [24] summarized the results of 11 randomized, placebo-controlled, open-label clinical trials of idebenone in Friedreich's disease (FD) from 1999 to 2008. The authors noted good tolerability of the drug at various stages of the disease in doses of up to 60 mg/kg per day. The results of a survey of 200 patients showed a positive effect of idebenone on both myocardial hypertrophy and neurological symptoms in FD.
The use of idebenone (Noben) for FD is being actively studied in our country. S.N. Illarioshkin and M.V. Ershov [3] examined 34 patients aged 7 to 59 years with a diagnosis of FD. All patients received Noben as a base drug at a dose of 5 mg/kg per day for 3 months. In most patients, the authors noted positive dynamics of the condition according to such clinical criteria as muscle strength in the limbs, tolerance to physical activity, general fatigue, motor activity, speech and coordination functions.
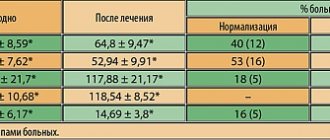
S.V. Kotov and O.P. Sidorov [4] investigated the use of idebenone in the treatment of Huntington's chorea. 18 patients with Huntington's chorea aged from 38 to 68 years were examined. The severity of the condition was assessed using a unified scale (maximum score - 28), cognitive functions were determined using the Mini Mental State Examination (MMSE, maximum score - 30). Noben was prescribed 90 mg per day (2 capsules of 30 mg in the morning and 1 capsule in the afternoon). Neurological status and cognitive functions were assessed before treatment and after 1, 3 and 6 months of therapy. Before taking Noben, in most cases, mild motor disorders were noted, the severity of choreic hyperkinesis was 10-11 points. All patients had a decrease in cognitive function; the average MMSE score before treatment was 21.2. After 1 month of taking the drug, the total MMSE score increased to 24, after 3 months it was 25.1 points, after 6 months it decreased slightly to 22.4 points (in 10 patients this indicator before treatment was 20.6 points). When studying individual indicators of the MMSE scale, improvements in orientation, memory, and counting were noted. In a number of patients, hyperkinesis decreased. The authors concluded that in patients with Huntington's chorea, Noben has a positive effect on cognitive function.
S. Shults et al. [29, 30] evaluated the safety and efficacy of an idebenone analogue (coenzyme Q10) in Parkinson's disease in a double-blind, placebo-controlled study. The study included 80 patients divided into 3 groups who took the drug at doses of 300, 600 and 1200 mg per day for up to 16 months, as well as 1200 IU of vitamin E per day. A statistically significant improvement in symptoms according to ADLS (Part II of the UPDRS scale) was obtained in the group of patients taking idebenone at a dose of 1200 mg per day, but the time to initiation of dopaminergic drug therapy did not increase.
T. Muller et al. [26] performed a double-blind, placebo-controlled study that included 28 patients with Parkinson's disease who received coenzyme Q10 at a dose of 360 mg per day for 4 weeks. A statistically significant improvement was obtained on the UPDRS scale in general, as well as color perception, but the therapy did not have a significant effect on motor symptoms. Moreover, in an open study by M. Horstink and B. van Engelen [16], which included 12 patients who took idebenone at a dose of 1000 mg per day for 3 months, and then 3 months at a dose of 1500 mg per day, a significant improvement in motor functions was shown in Parkinson's disease.
A systematic review from the Agency for Healthcare Research and Quality (AHRQ) and the McMaster University Center for Evidence-Based Practice [22] summarized the results of idebenone in 1153 patients with mild to moderate mixed dementia. The dose of the drug varied from 30 to 360 mg per day, the treatment interval ranged from 90 days to 60 weeks. Evidence was obtained of the positive effect of the drug on cognitive functions and global clinical assessment of patients. The qualitative score for reported adverse events ranged from 1 to 5. The discontinuation rate due to adverse events was 0-5% in the placebo group and 0-5% in the treatment group.
H. Gutzmann et al. [13, 14] compared the efficacy and safety of idebenone with the cholinergic drug tacrine. The study involved 203 patients from 40 to 90 years old, divided into 2 groups, one of which received idebenone at a dose of 360 mg per day (104 patients), the other received tacrine at a dose of 160 mg per day (99). Treatment was carried out for 60 weeks. To assess the effectiveness of therapy, the Efficacy Index Score (EIS) was used, including the state of cognitive functions, daily activity and other parameters.
By the end of the 60th week of therapy, 28.8%; of patients receiving idebenone, and only 9.1% of patients in the tacrine group continued to take prescribed medications; 50.0% of patients taking idebenone and 39.4% of patients taking tacrine showed improvement on the EIS and at least one of the secondary assessment scales. Thus, the authors noted better tolerability and effectiveness of idebenone compared to tacrine. In addition, a more favorable benefit-risk profile was obtained for idebenone compared to tacrine, which was also revealed when idebenone was compared with other acetylcholinesterase inhibitors.
Experimental studies have shown that in addition to the beneficial effects of idebenone on oxidative intracellular processes and stimulation of the formation of neurotrophic factors, the drug also normalizes the level of acetylcholine in various areas of the brain [18] and has disaggregant properties [33], which has an additional positive effect in case of cognitive impairment due to chronic vascular insufficiency.
Domestic researchers K.V. Voronkova and M.N. Meleshkov [1] when studying the effectiveness of Noben in dementia and moderate cognitive impairment showed the effectiveness of the drug. 35 patients aged from 60 to 86 years were observed. For dementia of the Alzheimer's type, mixed dementia and moderate cognitive impairment, Noben was used at a dose of 120 mg per day (in 2 doses) for 6 months. According to the General Clinical Impression scale, a positive effect of therapy was achieved in 13 (37%) patients, of which 5 had minimal improvement, 5 had moderate improvement, and 3 had pronounced improvement. In a detailed neuropsychological assessment, the effects of idebenone were found to improve both auditory-speech and visuospatial, short-term and delayed memory, as well as improve speech, including written language, and certain types of gnosis. In some cases, there was an improvement in mood.
Thus, idebenone (Noben) is a drug with well-studied pharmacokinetics and a proven efficacy and safety profile, which allows its use to be recommended for various diseases of the central nervous system.
Noben
Noben caps 30 mg x30 ATX code: N06BX13 (Idebenone)
Active substance: idebenone (idebenone) Rec.INN registered by WHO
Dosage form NOBEN® caps. 30 mg: 30 pcs.
reg. No.: LSR-005240/09 dated 06/30/09 - Registration period. beat is not limited
Release form, composition and packaging
Yellow capsules, size No. 1, the contents of the capsules are granulate containing granules and powder of yellow or yellow-orange color with orange and white inclusions.
1 caps. idebenone 30 mg
Excipients: lactose, microcrystalline cellulose, potato starch, low molecular weight povidone, magnesium stearate.
10 pieces. — cellular contour packages (1) — cardboard packs. 10 pieces. — cellular contour packages (2) — cardboard packs. 10 pieces. — contour cell packaging (3) — cardboard packs. 10 pieces. — contour cell packaging (6) — cardboard packs. 10 pieces. — contour cell packaging (12) — cardboard packs.
Clinical and pharmacological group: Nootropic drug
Pharmacotherapeutic group: Nootropic drug
pharmachologic effect
Pharmacokinetics
Indications
- psychoorganic syndrome caused by cerebral circulation disorders and age-related involutional changes in the brain,
- cerebroasthenic disorders of vascular, traumatic, psychogenic (neurasthenia) and combined etiology, manifested in the form of memory and/or attention impairments, decreased intellectual productivity and general activity, emotional instability, asthenic, astheno-depressive and moderately severe depressive states, headaches, dizziness , tinnitus.
ICD-10 codes
Dosage regimen
Prescribed orally 30 mg (1 capsule) 2-3 times a day. The duration of treatment is 1.5-2 months. Depending on the severity of symptoms, courses are repeated 2-3 times a year.
Capsules are taken after meals, the last dose is before 17:00.
Side effect
From the side of the central nervous system: difficulty falling asleep, mental agitation, headache.
Other: allergic reactions, dyspepsia.
Contraindications for use
- chronic renal failure,
- hypersensitivity to the components of the drug.
Use during pregnancy and breastfeeding
Use the drug with caution during pregnancy and lactation.
Use for renal impairment
Contraindicated in chronic renal failure.
special instructions
Impact on the ability to drive vehicles and operate machinery
It should not be taken while working by transport drivers and other persons whose profession requires a quick mental and motor reaction.
Overdose
Symptoms: increased severity of side effects.
Treatment: gastric lavage, taking activated charcoal, carrying out symptomatic therapy.
Drug interactions
No drug interactions have been established with Noben®.
Conditions for dispensing from pharmacies
The drug is available with a prescription.
Storage conditions and periods
List B. The drug should be stored out of the reach of children, in a dry place, protected from light at a temperature not exceeding 30°C. Shelf life: 3 years.