The action of nootropic drugs is aimed at improving cognitive abilities and brain functioning in general. Cereton belongs to this group of drugs. The drug provides metabolic protection and promotes the release of choline. This allows you to improve concentration, memory, and improve learning abilities. Against the background of the drug’s action, restoration processes are launched during the development of various pathologies.
pharmachologic effect
Nootropic. The active substance ensures the supply of choline to brain cells. The active ingredient is protected from enzymatic destruction. Choline is actively used in the process of synthesizing phosphatidylcholine and acetylcholine , normalizing the functioning of affected receptors, increasing the membrane elasticity of neuronal cells, and improving synaptic neuronal transmission.
With sufficient supply of choline to the brain, cerebral blood supply is enhanced, the reticular formation is activated, and cellular metabolism is stimulated. After a course of treatment, regression of severe neurological symptoms is recorded, blood circulation in the affected areas of the brain improves, and cognitive indicators improve. The medicine has a positive effect on the bioelectrical activity of the brain of spontaneous origin.
Special instructions and reviews
There are no data on interactions with other drugs. There is no effect on psychomotor functions, so there are no restrictions on driving a car or performing any operations requiring precision.
You can find both positive and negative reviews about the drug. Many patients emphasize that after the course of treatment, memory and concentration have significantly improved. Many also note a positive effect associated with slowing the progression of dementia.
All negative reviews, as a rule, contain information that there is no positive result. Negative side reactions of the body are also often observed, due to which it was necessary to abandon the medicine.
Indications for use of Cereton
- senile pseudomelancholia;
- cognitive impairment;
- attention disorder;
- dementia of unknown origin;
- encephalopathy of unspecified origin;
- cerebral infarction ;
- apathy;
- after ONMC ;
- encephalopathy;
- organic mental disorders;
- behavioral disorders of organic origin (with central nervous system dysfunction);
- senile dementia;
- lack of initiative, decreased motivation;
- lack of coordination;
- after intracranial hemorrhages;
- after brain injuries.
Additional indications for the use of Cereton in elderly people:
- psychoorganic syndrome,
- cerebrovascular insufficiency,
- multi-infarct dementia.
Side effects
Skin:
- hives;
- rashes.
Digestive tract:
- ulcerative lesions of the stomach;
- constipation;
- gastritis;
- pharyngitis;
- dry mouth mucous membranes.
Nervous system:
- dizziness;
- hyperkinesis;
- convulsive syndrome;
- nervousness;
- anxiety;
- aggression;
- drowsiness;
- migraine;
- ischemic changes in brain tissue.
Rarely, urinary disturbances, diarrhea syndrome , and pain in the injection area are recorded.
Contraindications and negative reactions of the body
The main contraindications to taking the drug are hypersensitivity to the active ingredient and other components. The medicine is not prescribed before reaching the age of 18 years, as well as during pregnancy and lactation.
Contraindications include the acute stage of hemorrhagic stroke, which, as a rule, develops against the background of high blood pressure.
When taking the drug, allergic reactions may occur: rash and itching on the skin. Other adverse reactions are rare. Nausea is sometimes observed after taking the drug, which is a consequence of the effect of the drug on the peripheral nervous system. When it appears, discontinuation of the drug is not required; it is enough to reduce the dosage.
It is necessary to consult a doctor when the medicine provokes disturbances in the functioning of the central nervous system. For example, during treatment, headaches, sleep problems, and increased excitability occur. It may also be necessary to discontinue the drug if gastritis and peptic ulcers worsen during oral administration, or severe constipation or diarrhea occurs.
Instructions for use of Cereton (Method and dosage)
Ampoules are prescribed mainly for acute conditions. The solution is administered intramuscularly or intravenously. Parenteral medication should be administered slowly. Daily dosage – 4 ml (1 ampoule).
The duration of treatment is 10-15 days.
After acute conditions, capsules are prescribed during the recovery period.
Instructions for use of Cereton tablets
2 pieces in the morning, 1 piece in the evening. Duration of therapy is up to 6 months. For cerebrovascular pathology of chronic origin and for dementia , 1 capsule is prescribed three times a day for a course of 3-6 months. The preferred time of administration is after meals.
Release form and composition
Cereton is produced in the form of gelatin capsules and solution for injection. The active substance in the drug is choline alfoscerate. Its content in capsules is 400 mg, in ampoules of 4 ml – 1000 mg.
The capsules are oval in shape and yellowish-brown in color. They are filled with an oily liquid. Auxiliary components used in the manufacture of capsules are glycerol and purified water. The capsule shell is made on the basis of gelatin using other additional components that allow it to be given the desired shape.
Analogues of Cereton
Level 4 ATC code matches:
Delecite
Choline alphoscerate
Cerepro
Gliatilin
Coincidence in pharmacological effects:
- Mexidol
- Actovegin
Structural analogues of Cereton:
- Gliatilin
- Cerepro
Cereton price, where to buy
The cost of Cereton solution is 3 ampoules - about 350 rubles, 5 ampoules - about 550 rubles. The price of Cereton in capsules is about 1000 rubles per pack of 28 pieces. In different regions, the price of Cereton varies depending on the pharmacy chain.
- Online pharmacies in RussiaRussia
- Online pharmacies in KazakhstanKazakhstan
ZdravCity
- Cereton solution for intravenous and intramuscular injection.
250 mg/ml 4 ml 5 pcs. CJSC “Pharm” 572 rub. order - Cereton capsules 400 mg 28 pcs. Artlife LLC Artlife/Sotex
RUB 1,047 order
- Cereton capsules 400 mg 56 pcs. Artlife/Sotex LLC
RUB 1,468 order
Pharmacy Dialogue
- Cereton (caps. 400 mg No. 28) Artlife LLC/PharmFirma Sotex CJSC
RUB 1,041 order
- Cereton (caps. 400 mg No. 56) Artlife LLC/PharmFirma Sotex CJSC
RUB 1,588 order
- Cereton (amp. 250 mg/ml 4 ml No. 3) Sotex
RUR 347 order
- Cereton (caps. 400 mg No. 14) Artlife LLC/PharmFirma Sotex CJSC
RUR 515 order
- Cereton (caps. 400 mg No. 28) Artlife LLC/PharmFirma Sotex CJSC
RUB 1,059 order
show more
Vascular diseases of the brain are not only a medical, but also a social problem, being one of the main causes of mortality and permanent disability [1]. Not only acute, but also chronic cerebrovascular accidents are widespread. Patients with such disorders make up a significant proportion of patients in neurological hospitals and at outpatient neurological and therapeutic appointments.
Chronic cerebral ischemia (CHI) is a slowly progressive disorder of cerebral circulation of a multifocal or diffuse nature, resulting from the gradual accumulation of ischemic and secondary degenerative changes in the brain caused by repeated ischemic episodes. Manifestations of CCI, depending on the stage of the process, can vary from subclinical phenomena to signs of persistent neurological deficit in combination with emotional, personal and cognitive disorders [2-4].
Cognitive impairment often accompanies acute and chronic vascular pathology of the brain [5-10]. At the initial stages of CCI, cognitive disorders are observed that do not cause social maladjustment. Currently, such disorders are referred to as “mild cognitive impairment.” General criteria for RBM were proposed in 1999 by R. Petersen et al. [11]: 1) complaints of increased forgetfulness or decreased mental performance; 2) information about a decrease in cognitive functions in comparison with the patient’s previously available capabilities; 3) objective evidence of mnestic or other cognitive impairments compared to the age norm; 4) cognitive impairment that does not lead to loss of professional abilities or social interaction skills (although there may be a slight deterioration in complex and instrumental types of daily and professional activities); 5) lack of grounds for a diagnosis of dementia.
A decrease in cerebral blood flow triggers a cascade of pathophysiological mechanisms leading to damage to brain tissue [12]. Currently obtained fundamental and clinical data allow us to consider the primary effects of cerebral ischemia to be a decrease in the delivery of the substrate of energy metabolism, a delayed excretion of lactic acid, neurotransmitters and toxic substances involved in the ischemic cascade [13]. In ischemic areas of the brain, energy deficiency, “switching” to anaerobic glycolysis, cell overload with calcium ions, metabolic acidosis, and oxidative stress consistently develop, which in turn leads to the oxidation of lipids and proteins in the cell membrane [14]. With CCI, phospholipase A2 is activated, which increases the degradation of phosphatidylcholine, reduces the level of cell phospholipids and, consequently, deteriorates the function of nerve cell membranes and impairs the transmission of nerve impulses. Along with a decrease in the intensity of synthesis, release and binding of acetylcholine, this pathogenetic mechanism underlies the development of cognitive impairment in CCI [15-17].
Timely diagnosis of the disease, elimination of existing vascular risk factors, adequate choice of therapeutic tactics, correction of cognitive impairment, helping to curb the increase in neuropsychological disorders, can significantly improve the prognosis of the disease and preserve patients’ ability to work for a long time [18].
The main directions of pathogenetic therapy for CCI are the correction of vascular risk factors, arterial hypertension, hyperlipidemia, the use of antiplatelet agents or anticoagulants, depending on the nature of the cardiovascular pathology. For stenotic lesions of the brachiocephalic arteries, in some cases, the use of reconstructive vascular interventions is effective [19, 20]. But despite a fairly wide arsenal of drugs proposed in recent years for the correction of mnestic disorders, their therapy presents significant difficulties [21].
Currently, several randomized studies have been conducted that were aimed at correcting vascular risk factors using antihypertensive drugs, using statins and glycemic control [22], which, however, did not lead to the expected results. Therefore, other groups of drugs are also being actively studied.
Most often in clinical practice, drugs from the following groups are used for CCI: 1) acetylcholinergic - acetylcholinesterase inhibitors, acetylcholine precursors; 2) reversible blocker of NMDA receptors; 3) nootropic; 4) vasoactive; 5) neurometabolic. It is believed that the use of drugs that help normalize metabolism in the brain and have neurotrophic and neuroprotective effects can be of significant importance in treatment tactics [13, 23].
One of the drugs with proven neuroprotective and neurotrophic effects is choline alfoscerate (Cereton) [24]. This drug, penetrating the blood-brain barrier, is broken down into choline and glycerophosphate. Choline is involved in the synthesis of the neurotransmitter acetylcholine, and glycerophosphate is a precursor of phosphatidylcholine, a component of neuronal membranes. Choline alfoscerate also has a dose-dependent stimulatory effect on the release of acetylcholine from the presynaptic cleft, demonstrating the pharmacological effect of a central cholinomimetic. Experimental studies have shown that choline alfoscerate helps to replenish acetylcholine deficiency in the central nervous system, stimulate cholinergic neurotransmission, and restore the plasticity of cell membranes [25]. In recent years, confirmation has been obtained that choline alfoscerate has a neuroprotective effect that is not directly related to the stimulation of cholinergic neurotransmission. Thus, in a model of spontaneously hypertensive rats, its ability to slow down the development of astrogliosis and prevent the destruction of phosphorylated neurofilaments was demonstrated, thus preventing the death of neurons [26].
Several randomized, double-blind clinical trials [27-29] provided evidence of the effectiveness of choline alfoscerate (Cereton) in patients with various types of cognitive disorders (Alzheimer's disease, vascular dementia, age-related cognitive decline); The effectiveness of ceretone in patients with Parkinson's disease with cognitive impairment was also noted [30]. It is believed [31] that cereton therapy is most effective at the stage of MCI.
The purpose of this study is to study the effectiveness and safety of treatment with Cereton for CCI with MCI.
Material and methods
The study included 25 patients, 16 women and 9 men, aged from 45 to 59 years (average - 53.8±1.3 years). Patients were observed both in inpatient and outpatient settings. All examined patients complained of decreased memory and mental performance.
The criteria for inclusion of patients in the study were: the presence of MCI and the absence of severe cognitive deficits (MMSE scores of at least 24 points); duration of complaints of memory loss for at least 3 months; the presence of vascular risk factors (arterial hypertension, diabetes mellitus, coronary heart disease, smoking); absence of severe depressive, anxiety or hypochondriacal disorders; age from 45 to 65 years; patient consent to participate in the study. Exclusion criteria were: the presence of severe somatic pathology; degenerative damage to the central nervous system (Parkinson's disease, Alzheimer's disease, dementia with Lewy bodies, etc.); use of other nootropic drugs, antidepressants, antiepileptic drugs up to 3 months before the start of the study; hypersensitivity to ceretone.
All patients underwent clinical general somatic, neurological, neuropsychological examinations, as well as the necessary instrumental and laboratory tests.
To assess the severity of cognitive disorders, we used the Mini-mental state examination (MMSE), the Hospital Anxiety and Depression Scale (HADS), and the SF-36 quality of life questionnaire, which provides its quantitative determination using scales of the physical and mental subjective well-being of patients (maximum satisfaction corresponds to a score of 100 points); the doctor and patient filled out a questionnaire for subjective assessment of effectiveness: “no effect,” “weak effect,” “moderate effect,” “pronounced effect.”
Cereton was prescribed at a dose of 1000 mg in 200 ml of saline (in the form of infusion) for 15 days in a hospital setting, then on an outpatient basis, 1 capsule (400 mg) 3 times a day for 3 months. All patients underwent neurological and neuropsychological examinations before and after the course of treatment. Additionally, at the end of the intravenous administration of the drug, the doctor and patient filled out a questionnaire for subjective assessment of effectiveness, and at the end of the 1st and 2nd months of therapy with ceretone capsules, the drug intake was monitored and information about possible adverse events was obtained, which was carried out by contacting the patient phone.
Statistical processing of the research results was carried out using the method of variation statistics and correlation analysis using the Microsoft Excel 7.0 software package.
Results and discussion
At the time of inclusion in the study, patients complained of memory loss, difficulty remembering, difficulty concentrating, headache, dizziness and unsteadiness when walking, general weakness and increased fatigue, and decreased mood. The distribution of these symptoms in patients with CCI is presented in Table. 1
, and the main neurological manifestations of the disease are given in
table.
2 .
According to magnetic resonance imaging (MRI) of the brain, various changes were identified in the study group of patients (Table 3)
.
By the 15th day of therapy, a subjective improvement in the patients’ condition was noted in the form of the appearance of “clearing in the head”, a decrease in the intensity of headaches, a decrease in the severity of noise in the head and unsteadiness when walking, an increase in mental performance and a decrease in asthenia. When assessing the effectiveness of cereton treatment by the patients themselves at the end of the study, all study participants noted an improvement. Of these, 48% reported significant improvement and 36% reported moderate improvement. The subjective clinical effect of cereton began to appear on days 5-7 from the start of infusions; this effect stabilized by the 15th day.
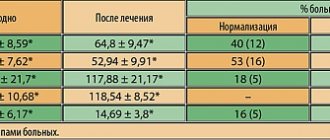
Summary data assessing the effectiveness of cereton treatment by patients and doctors on the 15th day of therapy and after its completion are presented in table. 4
.
At the end of the course of therapy in all patients there was a significant ( p
<0.05) the frequency and intensity of headaches, dizziness, noise in the head, fatigue decreased, and objective manifestations of emotional instability decreased.
By the end of treatment, objective neurological symptoms also changed. The dynamics of neurological symptoms before and after treatment with cereton showed a significant decrease in the frequency of pyramidal symptoms (from 64 to 36%; p
<0.05), nystagmus (from 36 to 16%;
p
<0.05) and pseudobulbar disorders (from 32 to 12 %;
p
<0.05).
As mentioned above, neuropsychological testing of patients was carried out at the beginning of the study and at the end of the study. The initial level of cognitive impairment on the MMSE scale was estimated at 25.6±1.2 points; when assessing the attention function according to Schulte tables, the rate of performance averaged 57.6 ± 1.3 s, according to the “10 words” test, immediate reproduction of 5.3 ± 0.5 words and delayed reproduction after 10 minutes of 3.3 ± 0 occurred, 5 words. At the end of treatment, the severity of cognitive impairment on the MMSE scale was 28.7±1.2 points, the rate of performance was 46.4±1.2 s, short-term memory (10-word test) was 7.9±0.4 words and delayed memory - 5.1±0.6 words.
When studying the level of anxiety on the HADS scale (Fig. 1)
in patients with CCI before treatment with cereton, it was noted that 24% of patients had no symptoms of anxiety (5.7±0.4 points), 52% had subclinically expressed anxiety (9.1±0.5 points), 24% - clinically expressed anxiety (13.2±0.4 points).
Figure 1. Indicators of the level of anxiety and depression on the HADS scale in patients at the beginning and end of treatment with Cereton. The y-axis is points; *- differences are significant compared to the initial value at p<0.05. When studying the level of depression on the HADS scale, symptoms of depression were absent in 40% of patients (6.1±0.1 points), subclinically expressed depression was in 52% (9.4±0.2 points), clinically pronounced depression was in 2 (8 %) of patients (12.7±0.2 points). At the end of the full course of treatment, there was a decrease in anxiety and depressive symptoms. Thus, reliably expressed symptoms of anxiety were absent in 44% of patients (4.9±0.3 points; p
<0.05), 44% had subclinically expressed anxiety (8.4±0.2 points), 12% had clinically expressed anxiety (11.9±0.3 points;
p
<0.05).
Symptoms of depression were absent in 60% of patients (4.8±0.2 points), subclinically expressed depression was in 36% (8.7±0.3 points; p
<0.05), clinically pronounced depression was in 1 (4% ) patient (12 points).
As a result of testing patients for all indicators of the SF-36 “quality of life” questionnaire, all studied quality of life parameters were significantly higher in patients at the end of the study compared to the day of inclusion; the average score of the overall quality of life indicator was above 50. Patients noted an increase in internal energy when performing daily duties, sensitivity to some manifestations of physical pain decreased, and it had less impact on behavior and the amount of work performed. At the end of treatment with Cereton, patients also rated their “general perception of health” and “vitality” significantly higher. They noted a significant increase in social activity. These data are shown in Fig. 2
and in
table.
5 .
Figure 2. Indicators of quality of life in patients at the beginning and end of the course of treatment with Cereton according to the SF-36 questionnaire, points. The x-axis is the SF-36 subscales: PF - physical activity; RP - the role of physical problems in limiting life activity; BP - physical pain; GH—general health perception; VT - viability; SF - social activity; RE—the role of emotional problems in disability; MH - mental health. The y-axis is points; *—differences are significant compared to the initial value at p<0.05; ** - at p<0.01.
During the study, side effects of the therapy were noted in 3 patients in the form of palpitations and dyspeptic disorders, which were moderately expressed and did not require cessation of treatment.
The data obtained indicate that the use of cereton in patients with CCI and MCI makes it possible to achieve positive dynamics in the patients’ condition, as evidenced by subjective improvement and objective data. A positive effect of cereton on the complex of clinical manifestations of this pathology was noted, with significant improvement in the areas of memory, concentration, flexibility of thinking and mental performance. The use of the drug is well tolerated and lacks severe side effects.
For the treatment of MCI in patients with CCI, it is recommended to carry out multi-stage therapy: parenterally for the first 10-15 days, then 1 capsule (400 mg) orally 3 times a day for 3-6 months. A course of use of the drug Cereton during the treatment of patients with CCI has a positive effect on their quality of life.
Cereton can be used in neurological and therapeutic departments of hospitals, as well as in outpatient practice to correct the clinical manifestations of CCI.