PHARMACOLOGICAL PROPERTIES
Pharmacodynamics. The main active ingredient of Okomistin® is
antiseptic benzyldimethyl-myristoylamino-propylammonium, which has a pronounced
antimicrobial effect against gram-positive and gram-negative, aerobic
and anaerobic bacteria in the form of monocultures and microbial associations, including hospital ones
strains with multidrug resistance to antibiotics. The drug acts on chlamydia,
pathogenic fungi, as well as herpes viruses, adenoviruses. The drug is more effective in
against gram-positive bacteria, including: staphylococci, streptococci.
Has an antifungal effect on ascomycetes of the genus Aspergillus and genus Penicillium,
yeast (Rhodotorula tubra, Torulopsis glabrata) and yeast-like (Trichophyton rubrum,
Trichophyton mentagrophytes, Trichophyton verrucosum, T. schoenleini, T. violaceum,
Epidermophyton Kaufman-Wolf, E, floccosum, Microsporum gypseum, Microsporum canis), and
also for other pathogenic fungi (for example, Pityrosporum orbiculare (Malassezia furfur)) in
in the form of monocultures and microbial associations, including fungal microflora with
resistance to chemotherapy drugs.
The action of benzyldimethyl-myristoylamino-propylammonium is based on direct
hydrophobic interaction of the molecule with the lipids of the membranes of microorganisms, leading
to their fragmentation and destruction. In this case, part of the benzyldimethylmyristoylaminopropylammonium molecule, plunging into the hydrophobic portion of the membrane, destroys
supra-membrane layer, loosens the membrane, increases its permeability for
large molecular substances, changes the enzymatic activity of the microbial cell,
inhibits enzyme systems, which leads to inhibition of vital activity
microorganisms and their cytolysis. Benzyldimethylmyristoylaminopropylammonium has
high selectivity of action against microorganisms, because practically no
acts on the membranes of human cells, which is due to the different structure of the latter -
significantly longer lipid radicals, sharply limiting the possibility
hydrophobic interaction of benzyldimethyl-myristoylamino-propylammonium with cells.
Under the influence of the drug, the resistance of bacteria and fungi to antibiotics is reduced.
Benzyldimethylmyristoylaminopropylammonium has anti-inflammatory and
immunoadjuvant effect, enhances local protective reactions, regenerative
processes, activates nonspecific defense mechanisms due to modulation
cellular and local humoral immune response.
Pharmacokinetics. The drug has a local effect. Possible data
there are no penetrations into the bloodstream.
Antimicrobial eye drops
Used only for bacterial conjunctivitis. Typical signs are redness, the appearance of purulent, sometimes profuse yellowish discharge, sticking together the edges of the eyelids and eyelashes [2, 3].
Over-the-counter drugs
Sulfacetamide (albucid)
Very popular drops from the Soviet past. The active substance belongs to the group of sulfonamides. The drug is active against gram-positive and gram-negative cocci, as well as some gram-negative microorganisms and even intracellular ones - in particular, chlamydia. However, many bacteria develop resistance to sulfacetamide, and therefore its effectiveness is sharply reduced.
Available in two forms - 10% (children from 0) and 20% (children over 2 months and adults).
Sulfacetamide drops should be applied frequently, every 2-3 hours (that is, 6-8 times a day). As you recover, the frequency of use may be reduced. When instilling drops, a burning sensation may be felt.
The drug must be stored in the refrigerator: the instructions for some medications require storage at a temperature no higher than 15 °C, and others at no higher than 25 °C.
Chloramphenicol
Being a broad-spectrum antibiotic, it is active against most microorganisms that cause damage to the conjunctiva, including staphylococci, streptococci and Haemophilus influenzae.
NB! Some chloramphenicol TN in the form of eye drops belong to the Rx group. Before dispensing a drug, you should carefully study the instructions to make sure that a particular product can be sold without a doctor's prescription.
Chloramphenicol antimicrobial eye drops should be stored in the refrigerator (temperature 8–15 °C) [4].
Prescription drugs
The list of prescription antibacterial eye medications with antibacterial effects is quite long. Most drugs are fluoroquinolones. Their spectrum of activity covers the majority of microorganisms associated with bacterial inflammatory diseases of the conjunctiva [5].
Today, the following INNs for antibacterial eye drops are registered in the Russian Federation:
- Macrolides - azithromycin;
- Fluoroquinolones - norfloxacin, ofloxacin, gatifloxacin, levofloxacin, lomefloxacin, moxifloxacin, ciprofloxacin;
- Aminoglycosides - azithromycin, tobramycin;
- Fusidic acid.
To reduce the risk of bacteria developing drug resistance, it is important to strictly follow your doctor's instructions regarding the frequency and duration of use of eye drops.
INDICATIONS FOR USE
Ophthalmology
Okomistin®, eye, ear, nasal drops are recommended for use in complex
treatment of infectious processes in the anterior part of the eye (blepharitis, conjunctivitis,
keratitis, keratouveitis), eye injuries, eye burns (thermal and chemical); V
preoperative and postoperative periods for the treatment and prevention of purulent inflammatory eye lesions.
Prevention of ophthalmia of newborns, including gonococcal and chlamydial.
Otorhinolaryngology
The drug is used in the complex treatment of acute sinusitis/rhinosinusitis, exacerbation
chronic sinusitis/rhinosinusitis, acute rhinitis; acute and chronic external
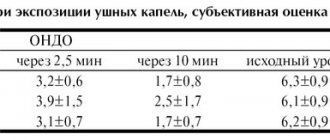
otitis, chronic purulent mesotympanitis, otomycosis.
Types of conjunctivitis
First of all, it is important to understand that for any ophthalmological problems, a doctor’s consultation is necessary, and the client should be warned about this first. There are various pathological conditions for which drugs with radically different mechanisms of action are prescribed [1]:
- Bacterial conjunctivitis associated with bacterial pathogens. Most often, it is bacteria that cause inflammatory processes in the conjunctiva. The basis of treatment is local antiseptic and antibacterial eye drops. The discharge from the eye is pus.
- Viral conjunctivitis caused by the development of a viral infection, usually adenoviruses [1]. The basis of therapy is antiviral eye drops. The discharge from the eye does not have the character of pus.
- Allergic conjunctivitis. First-line drugs are systemic and local antihistamines and intranasal corticosteroids, and less commonly, cromoglycates. There are other symptoms of hay fever.
Of course, the primary care physician should not engage in differential diagnosis and search for the causes of the disease, but he can and should recommend anti-inflammatory eye drops of the OTC group.
It is necessary to clearly distinguish between medications that are used for different types of inflammation of the conjunctiva. We talked about drops that are used for allergic conjunctivitis quite recently. This material will discuss drugs used to treat inflammation of the conjunctiva of the eye, viral and bacterial conjunctivitis.
What should I warn the client about?
Terms of use.
To ensure that the drops remain sterile after opening, it is important not to touch the tip of the tube to the eye during use and to close it tightly after each use. In addition, it should be borne in mind that the shelf life of eye drops after opening may be limited: this information will certainly be in the instructions for use.
Storage conditions.
A common mistake made by many consumers is storing all eye drops in the refrigerator. In fact, drugs in this group do not always have to be stored at a cool temperature, including after opening. Be sure to draw the visitor’s attention to this, warning how exactly specific drops should be stored.
Drugs used to treat bacterial conjunctivitis can be divided into 4 categories:
- Antimicrobial;
- Antiseptic;
- Corticosteroids;
- Combined.
Consumer choice and the doctor's recommendations are limited to the first two subgroups, which include over-the-counter drugs.
METHOD OF APPLICATION AND DOSES
Ophthalmology
Locally. For adults, for therapeutic purposes, the drug Okomistin® is instilled into the conjunctival
bag 1-2 drops 4-6 times a day until clinical recovery.
For prophylactic purposes, the drug is instilled 2-3 days before surgery, as well as during
10-15 days after surgery, 1-2 drops 3 times a day.
When treating eye burns, after washing the eye with plenty of water,
frequent instillations (every 5-10 minutes) for 1-2 hours. For further treatment
the drug is used 1-2 drops 4-6 times a day.
In pediatric practice, for the treatment of bacterial conjunctivitis in children, the drug
Okomistin® is instilled into the conjunctival sac, 1 drop up to 6 times a day for
7-10 days.
To prevent ophthalmia in newborns, the child is given instillation immediately after birth.
1 drop of the drug in each eye 2 times with an interval of 2-3 minutes.
Otorhinolaryngology
Locally. Adults with acute sinusitis/rhinosinusitis, exacerbation of chronic
sinusitis/rhinosinusitis, acute rhinitis, infections of the nasal mucosa drug
Okomistin® is instilled 2-3 drops into each nostril, 4-6 times a day. Course of treatment up to 14
days.
In pediatric practice, for the treatment of acute rhinosinusitis, exacerbation of chronic
rhinosinusitis in children over 1 year of age, the drug Okomistin® is instilled into each nasal passage
1-2 drops up to 4-6 times a day for 10-14 days.
For adults. Locally.
For acute and chronic external otitis, otomycosis, the drug Okomistin® is instilled into
external auditory canal 5 drops 4 times a day or, instead of instillation, into the external
gauze turunda soaked in the drug is introduced into the ear canal 4 times a day. Well
treatment is 10 days.
In adults with chronic mesotympanitis, it is used in complex treatment with
using hardware ultrasonic irrigation or insertion into the tympanic cavity
together with antibiotics.
In the absence of positive dynamics (increased severity or appearance
new signs/symptoms of the disease, the occurrence of complications) on the 3-4th day of therapy with
When using the drug Okomistin®, you must consult a doctor!
Side effects Allergic reactions are possible. In some cases it may
there may be a slight burning sensation, discomfort that goes away on its own
15-20 seconds and does not require discontinuation of the drug.
If any adverse events occur, you should consult a doctor.
Instructions for medical use of the drug Okomistin® (Okomistin®)
Registration number LSR-004896/09
INN or group name Benzyldimethyl-myristoylamino-propylammonium
Chemical name Benzyldimethyl[3-(myristoylamino)propyl]ammonium chloride monohydrate
Dosage form Eye, ear, nasal drops
Composition per 100 ml Active substance: Benzyldimethyl[3-(myristoylamino)propyl] ammonium chloride monohydrate (in terms of anhydrous substance) - 0.01 g
Excipients: sodium chloride - 0.90 g, purified water up to 100 ml
Description of the dosage form
Colorless transparent liquid
Pharmacotherapeutic group Antiseptic
ATX code S01AX
Pharmacological properties
Pharmacodynamics.
The main active ingredient of the drug Okomistin® is the antiseptic benzyldimethyl-myristoylamino-propylammonium, which has a pronounced antimicrobial effect against gram-positive and gram-negative, aerobic and anaerobic bacteria in the form of monocultures and microbial associations, including hospital strains with multidrug resistance to antibiotics. The drug acts on chlamydia, pathogenic fungi, as well as herpes viruses and adenoviruses. The drug is more effective against gram-positive bacteria, including: staphylococci, streptococci.
It has an antifungal effect on ascomycetes of the genus Aspergillus and the genus Penicillium, yeasts (Rhodotorula tubra, Torulopsis glabrata) and yeasts (Trichophyton rubrum, Trichophyton mentagrophytes, Trichophyton verrucosum, T. schoenleini, T. violaceum, Epidermophyton Kaufman-Wolf, E, floccosum, Microsporum gypseum , Microsporum canis), as well as other pathogenic fungi (for example, Pityrosporum orbiculare (Malassezia furfur)) in the form of monocultures and microbial associations, including fungal microflora with resistance to chemotherapeutic drugs.
The action of benzyldimethyl-myristoylamino-propylammonium is based on the direct hydrophobic interaction of the molecule with the lipids of the membranes of microorganisms, leading to their fragmentation and destruction. In this case, part of the benzyldimethyl-myristoylamino-propylammonium molecule, plunging into the hydrophobic portion of the membrane, destroys the supra-membrane layer, loosens the membrane, increases its permeability for large-molecular substances, changes the enzymatic activity of the microbial cell, inhibits enzyme systems, which leads to inhibition of the vital activity of microorganisms and their cytolysis. Benzyldimethyl-myristoylamino-propylammonium has a high selectivity of action against microorganisms, because has virtually no effect on the membranes of human cells, which is due to the different structure of the latter - the significantly longer length of lipid radicals, which sharply limits the possibility of hydrophobic interaction of benzyldimethyl-myristoylamino-propylammonium with cells. Under the influence of the drug, the resistance of bacteria and fungi to antibiotics is reduced. Benzyldimethylmyristoylaminopropylammonium has anti-inflammatory and immunoadjuvant effects, enhances local protective reactions, regenerative processes, activates nonspecific defense mechanisms due to modulation of the cellular and local humoral immune response.
Pharmacokinetics . The drug has a local effect. There are no data on possible penetration into the bloodstream.
Indications for use
Ophthalmology
Okomistin®, eye, ear, and nasal drops are recommended for use in the complex treatment of infectious processes in the anterior part of the eye (blepharitis, conjunctivitis, keratitis, keratouveitis), eye injuries, eye burns (thermal and chemical); in the preoperative and postoperative periods for the treatment and prevention of purulent-inflammatory eye lesions. Prevention of ophthalmia of newborns, including gonococcal and chlamydial.
Otorhinolaryngology
The drug is used in the complex treatment of acute sinusitis/rhinosinusitis, exacerbation of chronic sinusitis/rhinosinusitis, acute rhinitis; acute and chronic external otitis, chronic purulent mesotympanitis, otomycosis.
Contraindications
Individual sensitivity to the components of the drug.
Use during pregnancy and breastfeeding
The use of the drug during pregnancy and lactation is possible only if the expected benefit to the mother outweighs the potential risk to the fetus and child.
Directions for use and doses
Ophthalmology
Locally. For adults, for therapeutic purposes, the drug Okomistin® is instilled into the conjunctival sac, 1-2 drops 4-6 times a day until clinical recovery. For prophylactic purposes, the drug is instilled 2-3 days before surgery, as well as for 10-15 days after surgery, 1-2 drops 3 times a day. When treating eye burns, after washing the eye with plenty of water, perform frequent instillations (every 5-10 minutes) for 1-2 hours. For further treatment, the drug is used 1-2 drops 4-6 times a day. In pediatric practice, for the treatment of bacterial conjunctivitis in children, the drug Okomistin® is instilled into the conjunctival sac, 1 drop up to 6 times a day for 7-10 days. To prevent ophthalmia in newborns, immediately after birth, the child is instilled with 1 drop of the drug into each eye 2 times with an interval of 2-3 minutes.
Otorhinolaryngology
Locally. For adults with acute sinusitis/rhinosinusitis, exacerbation of chronic sinusitis/rhinosinusitis, acute rhinitis, infection of the nasal mucosa, Okomistin® is instilled 2-3 drops into each nostril, 4-6 times a day. The course of treatment is up to 14 days. In pediatric practice, for the treatment of acute rhinosinusitis, exacerbation of chronic rhinosinusitis in children over 1 year of age, the drug Okomistin® is instilled into each nasal passage, 1-2 drops up to 4-6 times a day for 10-14 days. For adults. Locally. For acute and chronic external otitis and otomycosis, the drug Okomistin® is instilled into the external auditory canal 5 drops 4 times a day or, instead of instillation, a gauze turunda soaked in the drug is injected into the external auditory canal 4 times a day. The course of treatment is 10 days. In adults with chronic mesotympanitis, it is used in complex treatment using hardware ultrasonic irrigation or injection into the tympanic cavity together with antibiotics. In the absence of positive dynamics (increased severity or appearance of new signs/symptoms of the disease, the occurrence of complications) on the 3-4th day of therapy using the drug Okomistin®, you must consult a doctor!
Side effect
Allergic reactions are possible. In some cases, a slight burning sensation and discomfort may occur, which go away on their own after 15-20 seconds and do not require discontinuation of the drug. If any adverse events occur, you should consult a doctor.
Interaction with other drugs
When used together, Okomistin® increases the effectiveness of topical antibiotics. Interaction studies of the drug with other drugs have not been conducted.
special instructions
Contact lenses should be removed immediately before instillation of Okomistin® and put on no earlier than 15 minutes after instillation. To avoid contamination and cross-infection, do not use the same vial to treat eye, nose, and/or ear infections at the same time. To prevent contamination of the drug solution during instillation, patients should avoid contact of the dropper tip with the eye and skin. Sharing the dropper bottle by more than one person may spread the infection.
Children
The drug can be used in pediatric practice.
Overdose
Not observed.
Impact on the ability to drive vehicles and machinery
After using the drug, a temporary decrease in the clarity of visual perception is possible and until it is restored, it is not recommended to drive a car or engage in activities that require increased attention and reaction.
Release form
Okomistin®, eye, ear, nasal drops, 1 ml or 1.5 ml in disposable polymer tube-droppers, 5 or 10 ml in polymer bottles, sealed with a dropper stopper and a screw-on cap with first opening control; 20 ml in glass bottles, sealed with a rubber stopper and an aluminum cap or a polyethylene dropper cap. 1 or 5 bottles or 5 or 10 dropper tubes with instructions for use in a cardboard pack.
Storage conditions Store at a temperature not exceeding 25°C. Keep out of the reach of children.
Shelf life - 3 years. Shelf life after opening is 1 month. Do not use after expiration date.
Conditions for dispensing from pharmacies
Dispensed with a doctor's prescription.
Registration Certificate Holder
LLC "INFAMED" 142700, Russia, Moscow region, Leninsky district, Vidnoye, ter. Industrial zone of OJSC VZ GIAP, building 473, floor 2, room. 9, tel.
Manufacturer
LLC "INFAMED K" 238420, Russia, Kaliningrad region, Bagrationovsky district, Bagrationovsk, st. Communalnaya, 12, tel.
Organization authorized to accept complaints
LLC "INFAMED" 142700, Russia, Moscow region, Leninsky district, Vidnoye, ter. Industrial zone of OJSC VZ GIAP, building 473, floor 2, room. 9, tel.
special instructions
Contact lenses should be removed immediately before instillation of the drug.
Okomistin® and put on no earlier than 15 minutes after instillation.
To avoid contamination and cross-infection, do not
Use one bottle to simultaneously treat an infection of the eye, nose and/or ear. For
To prevent contamination of the drug solution during instillation, patients should
Avoid contact of the tip of the dropper with the eye and skin. Using a dropper bottle
by more than one person can lead to the spread of infection.
Children
The drug can be used in pediatric practice.
Release form
Okomistin®, eye, ear, nasal drops, 1 ml or 1.5 ml in disposable polymer
dropper tube, 5 or 10 ml in polymer bottles, sealed with a dropper stopper and a screw-on cap with first opening control; 20 ml in glass
bottles sealed with a rubber stopper and an aluminum or polyethylene cap
dropper cap.
1 or 5 bottles or 5 or 10 dropper tubes with instructions for use per pack
from cardboard.