Not to be confused with Somatocrinin or Somatomedin.
| SST | |||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||
| Nicknames | SST, SMST, somatostatin, somatostatin, somatostatin | ||||||||||||||||||||||||||||||||||
| External identifiers | OMIM: 182450 MGI: 98326 HomoGene: 819 Gene maps: SST | ||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||
| Orthologs | |||||||||||||||||||||||||||||||||||
| Variety | Human | Mouse | |||||||||||||||||||||||||||||||||
| Entrez |
| ||||||||||||||||||||||||||||||||||
Production
Digestive system
Somatostatin is secreted by delta cells in several places in the digestive system, namely the pyloric antrum, the duodenum and the pancreatic islets.[13]
Somatostatin released into the pyloric antrum passes through the portal venous system to the heart, then enters the systemic circulation and reaches those places where it will have an inhibitory effect. Additionally, the release of somatostatin from delta cells may act in a paracrine manner.[13]
In the stomach, somatostatin acts directly on acid-producing parietal cells through a G protein-coupled receptor (which inhibits adenylate cyclase, thus effectively counteracting the stimulatory effect of histamine) to reduce acid secretion.[13] Somatostatin may also indirectly reduce acid production in the stomach by preventing the release of other hormones, including gastrin and histamine, which effectively slow down the digestive process.
Brain
| Sst is expressed in mouse telencephalon interneurons at embryonic day 15.5. Allen's Brain Atlases | Sst expression in the adult mouse. Allen's Brain Atlases |
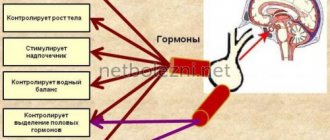
Somatostatin is produced by neuroendocrine neurons in the ventromedial nucleus of the hypothalamus. These neurons project to the median eminence, where somatostatin is released from the nerve endings into the hypothalamic-pituitary axis through the neuronal axons. Somatostatin is then transported to the anterior pituitary gland, where it suppresses the secretion of growth hormone from the growth hormone cells. Somatostatin neurons in the periventricular nucleus mediate the negative feedback effects of growth hormone on its own release; Somatostatin neurons respond to high circulating concentrations of growth hormone and somatomedins by increasing the release of somatostatin, thereby decreasing the rate of growth hormone secretion.
Somatostatin is also produced by several other populations that project centrally to other brain regions, and somatostatin receptors are expressed in many different brain regions. In particular, populations of somatostatin neurons occur in the arcuate nucleus, [ citation needed
] then the hippocampus,[
citation needed
] and the brainstem nucleus of the solitary tract.[
citation needed
]
Clinical observations
Long-acting octreotide relieves clinical manifestations in patients with NETs and has antitumor activity in patients with metastatic intestinal NETs, but it remains unclear whether it has antiproliferative effects in patients with advanced pancreatic NETs. H. Kitade et al. (2018) reported a 71-year-old man with multiple liver metastases from pancreatic NETs who was treated with everolimus 10 mg and sunitinib 25 mg daily for 3 weeks. However, therapy was discontinued due to disease progression and serious side effects. Extended-release octreotide was initially administered simultaneously with everolimus; the level of tumor markers decreased markedly, and liver metastases decreased in size. Immunohistochemical examination of tumor samples obtained before treatment showed a high level of expression of somatostatin receptor type 2. Clinical observation suggested that long-acting octreotide may be an option for the treatment of advanced pancreatic NETs after discontinuation of everolimus and sunitinib [30].
T. Arao et al. (2017) presented a 54-year-old woman hospitalized for treatment of acromegaly. Growth hormone (GH) levels were 39.8 ng/mL, insulin-like growth factor-1 (IGF-1) levels were 717 ng/mL, and GH levels were not suppressed (22.9 ng/mL) during an oral glucose tolerance test. MRI showed that the tumor, measuring 21x17 mm, extended into the right suprasellar region and invaded the cavernous sinus. Treatment with long-acting octreotide was started at a dose of 20 mg every 4 weeks. After three doses, GH and IGF-1 levels decreased to 2.19 ng/ml (to 1.69 after glucose tolerance test) and 205 ng/ml, respectively. A decrease in GH and IGF-1 levels, a decrease in tumor size and cavernous sinus invasion allowed surgical treatment to be performed without subsequent complications. Preoperative administration of long-acting octreotide improved outcomes in the treatment of acromegaly [31].
Pregnancy with acromegaly occurs rarely and proceeds favorably, but during this period an increase in tumor size may occur. Correcting tumor growth during pregnancy is difficult due to the potential complications of possible surgery and the side effects of anticancer drugs. AM Hannon et al. (2019) described a unique case of octreotide use in a 32-year-old woman with acromegaly diagnosed at 11 weeks. pregnancy. There was a large macroadenoma penetrating into the suprasellar cistern at 20 weeks. Bitemporal hemianopsia developed during pregnancy. The patient refused surgery and was prescribed treatment with octreotide 100 mcg 3 times a day subcutaneously, which led to normalization of visual fields after 2 weeks. therapy. Further decrease in visual fields at 24 weeks. pregnancy forced an increase in the dose of octreotide to 150 mcg 3 times a day; vision remained stable until the end of pregnancy. At 14 weeks. Gestational diabetes was diagnosed, and from the 22nd week. insulin was prescribed. There were no other obstetric complications, and fetal growth continued until the end of pregnancy. A planned caesarean section was performed at 34 weeks, fetal weight at birth was 3.2 kg, Apgar score 9, no congenital anomalies were detected. The menstrual cycle after childbirth is regular. The woman was prescribed extended-release octreotide at a dose of 40 mg and referred for surgery. 2 years after birth, the child was developing normally. The occurrence of acromegaly during pregnancy and the improvement of perimetry parameters with octreotide were first described [32].
Giant pituitary adenomas are benign tumors ≥4 cm in diameter. They present with symptoms due to hypersecretion of one or more pituitary hormones and involvement of surrounding structures in growth, and compression of the pituitary gland can lead to hypopituitarism. F. Dicuonzo et al. (2019) reported a young woman with acromegaly with an unresectable giant GH-secreting pituitary adenoma extending to the cavernous sinus, orbital cavity, maxillary sinus, sphenoid sinus, and right temporal fossa. Treatment with long-acting octreotide helped to quickly relieve headaches and bilateral hemianopia resulting from involvement of the optic chiasm, reduce the clinical manifestations of acromegaly and control tumor growth over time, enhance fertility, and allowed the patient to become pregnant. Therapy was discontinued during pregnancy. The patient gave birth to a healthy son. Tumor size did not increase at the end of pregnancy and during follow-up. This case confirms the possibility of a favorable pregnancy outcome in a patient suffering from acromegaly [33].
Ectopic adrenocorticotropic hormone (ACTH) syndrome is a rare disease often accompanied by severe hypercortisolism requiring surgical intervention. P. Rodrigues et al. (2012) described a 33-year-old patient hospitalized in 1993 with clinical manifestations of Cushing's syndrome. He had high plasma ACTH levels and dramatically increased urinary free cortisol excretion, which were not suppressed by high doses of dexamethasone. The pituitary gland appeared normal on MRI, but a chest CT scan revealed a 1.7 cm mass in the left lung. Instrumental studies showed the presence of ACTH-producing neuroendocrine carcinoma with distant metastases. The patient received chemotherapy plus octreotide, which resulted in a favorable clinical and biochemical outcome. The authors highlight the long-term response to drug therapy with octreotide with excellent antiproliferative effects [34].
A. Kanno et al. (2017) presented a case of insulinoma in an 80-year-old woman with episodes of hypoglycemia. The patient refused surgical treatment; subcutaneous administration of 50 mcg of octreotide at bedtime prevented hypoglycemia at night. The drug is capable of suppressing insulin secretion, although there is no specific protocol for its use in such cases. The patient was unable to administer the drug herself daily; she was prescribed long-acting octreotide, which prevented episodes of hypoglycemia for 4 weeks, and the patient’s quality of life improved significantly [35].
Functions
Cell D is shown at the top right, and somatostatin is represented by the middle arrow pointing to the left.
Somatostatin is classified as an inhibitory hormone,[6] and is induced by low pH.[ citation needed
] Its effect spreads to different parts of the body. The release of somatostatin is inhibited by the vagus nerve.[14]
Anterior pituitary gland
in the anterior pituitary gland, effects of somatostatin:
- Suppressing growth hormone (GH) release[15] (thus counteracting the effects of growth hormone releasing hormone (GHRH))
- Inhibiting the release of thyroid-stimulating hormone (TSH)[16]
- Inhibition of adenylyl cyclase in parietal cells
- Inhibition of prolactin release (PRL)
Gastrointestinal system
- Somatostatin is homologous to cortistatin (see somatostatin family) and inhibits the release of gastrointestinal hormones
- Decreases the rate of gastric emptying and reduces smooth muscle contraction and intestinal blood flow.[15]
- Suppresses pancreatic hormone release Somatostatin release is triggered by the beta cell peptide urocortin3 (Ucn3) to inhibit insulin release.[17][18]
- Inhibits the release of glucagon[17]
Synthetic substitutes
| This section need additional quotes for verification . |
Octreotide (brand name Sandostatin, Novartis Pharmaceuticals) is an octapeptide that pharmacologically mimics natural somatostatin, but is a more potent inhibitor of growth hormone, glucagon and insulin than the natural hormone, and has a much longer half-life (about 90 minutes compared to 2–3 minutes for somatostatin). Since it is poorly absorbed from the intestine, it is administered parenterally (subcutaneously, intramuscularly or intravenously). It is indicated for the symptomatic treatment of carcinoid syndrome and acromegaly. It is also increasingly used in polycystic liver and kidney diseases.
Lanreotide (Somatulin, Ipsen Pharmaceuticals) is a medication used to treat acromegaly and symptoms caused by neuroendocrine tumors, primarily carcinoid syndrome. It is a long-acting somatostatin analogue like octreotide. It is available in several countries, including the UK, Australia and Canada, and was approved for sale in the US by the Food and Drug Administration on August 30, 2007.
Pasireotide, sold under the brand name Signifor, is an orphan drug approved in the US and European Union for the treatment of Cushing's disease in patients who have not undergone or are not suitable for surgical treatment. It was developed by Novartis. Pasireotide is a somatostatin
an analogue with a 40-fold increase in affinity for somatostatin receptor 5 compared to other somatostatin analogues.
Introduction
In recent decades, the incidence of neuroendocrine tumors (NETs) has been steadily increasing.
NETs are a relatively rare heterogeneous group of neoplasms with an annual incidence of 35 cases per 100,000 people. The updated WHO classification of gastroenteropancreatic (GEP) tumors verifies them depending on location, clinical manifestations and degree of differentiation. Due to their slow growth and lack of early symptoms, most NETs are often diagnosed at advanced stages, when treatment options are limited [1]. NETs occur in any organ where endocrine cells are present. These neoplasms may not produce clinical symptoms, but if they are functional, endocrine syndromes develop that pose a threat to the health and life of patients.
The goal of treating patients with NET is to eliminate the tumor itself and suppress the symptom complex caused by carcinoid syndrome. The only radical method of treatment remains surgical, and if it is impossible to use, inhibition of tumor growth and suppression of hormonal expression with somatostatin analogues (in particular, octreotide) can prolong life and improve its quality in patients.
Evolutionary history
Six somatostatin genes have been discovered in vertebrates. The currently proposed origin story for these six genes is based on three whole-genome duplication events that occurred in vertebrate evolution, along with local duplications in vertebrates. bonyness of fish. The ancestral somatostatin gene was duplicated during the first whole genome duplication event (1R) to create SS1
and
SS2
.
These two genes were duplicated during a second whole-genome duplication event (2R) to create four new somatostatin genes: SS1, SS2, SS3
, and one gene lost during vertebrate evolution.
Tetrapods retained SS1
(also known as
SS-14
and
SS-28
) and
SS2
(also known as cortistatin) after the split in Sarcopterygia and Actinopterygia split the lineage.
In teleost fish, SS1, SS2
, and
SS3
were duplicated during the third whole-genome duplication event (3R) to create
SS1, SS2, SS4, SS5,
and two genes that were lost during the evolution of teleost fish.
SS1
and
SS2
went through local duplication to call
SS6
and
SS3
.[8]
Recommendations
- ^ a b c
GRCh38: Ensemble issue 89: ENSG00000157005 — Ensemble, May 2017 - ^ a b c
GRCm38: Ensemble Issue 89: ENSMUSG00000004366 — Ensemble, May 2017 - "Human's Guide to PubMed:". National Center for Biotechnology Information, US National Library of Medicine
. - "Mouse PubMed link:". National Center for Biotechnology Information, US National Library of Medicine
. - "somatostatin". Encyclopædia Britannica. Encyclopedia Britannica Online. Encyclopedia Britannica Inc., 2021. Online. 04 mag. 2016 https://www.britannica.com/science/somatostatin>.
- ^ a b
Kostoff A. "Section 5, Chapter 4: Structure, synthesis and secretion of somatostatin."
Endocrinology: endocrine pancreas
. Medical College of Georgia. paragraph 16. Archived from the original on April 5, 2008. Retrieved 2008-02-19. - "somatostatin preproprotein." NCBI reference sequence
. National Center for Biotechnology Information (NCBI). - ^ a b c
Liu Y, Liu D, Zhang Y, Li S, Liu X, Lin X (September 2010).
"Evolution of somatostatin in vertebrates". Gen.
_
463
(1–2):21–8. doi:10.1016/j.gene.2010.04.016. PMID 20472043. - Gajete, MD, Cordova-Chacón J, Duran-Prado, M, Malagon, M.M., Martinez-Fuentes, A.J., Gracia-Navarro, F., Luque, R.M., Castaño, J.P. (July 2010). "Somatostatin and its receptors from fish to mammals". Annals of the New York Academy of Sciences
.
1200
: 43–52. Doi:10.1111/j.1749-6632.2010.05511.x. PMID 20633132. S2CID 23346102. - "Entrez Jean: somatostatin."
- Shen LP, Pictet RL, Rutter WJ. (August 1982). "Human somatostatin I: cDNA sequence". Proceedings of the National Academy of Sciences of the United States of America
.
79
(15):4575–9. doi:10.1073/psas.79.15.4575. PMC 346717. PMID 6126875. - Shen LP, Rutter WJ (April 1984). "Sequence of the human somatostatin I gene". The science
.
224
(4645): 168–71. Doi:10.1126/science.6142531. PMID 6142531. - ^ a b c
Bohr WF, Boulpaep EL (2012).
Medical physiology
(2nd ed.). Philadelphia, PA: Elsevier. ISBN 9781437717532. - Holst JJ, Skak-Nielsen T, Orskov S, Seier-Poulsen S (August 1992). "Vagulatory control of somatostatin, vasoactive intestinal polypeptide, gastrin-releasing peptide, and HCl release from the nonantral pig stomach." Scandinavian Journal of Gastroenterology
.
27
(8): 677–85. Doi:10.3109/00365529209000139. PMID 1359631. - ^ a b
Bowen R. (2002-12-14).
"Somatostatin". Biomedical Hypertexts
. Colorado State University. Retrieved 2008-02-19. - First Aid for USMLE Step 1, 2010. Pg. 286.
- ^ a b
Kostoff A. "Section 5, Chapter 4: Structure, synthesis and secretion of somatostatin."
Endocrinology: endocrine pancreas
. Medical College of Georgia. paragraph 17. Archived from the original on March 31, 2008. Retrieved 2008-02-19. - van der Meulen T., Donaldson S.J., Caceres E., Hunter A.E., Cowing-Citron S., Pound L.D., Adams M.W., Ziebrzycki A., Grove K.L., Huizing, M. O. (July 2015). "Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion." Nature Medicine
.
21
(7): 769–76. doi:10.1038/nm.3872. PMC 4496282. PMID 26076035.
further reading
- Florio T., Schettini G. (September 2001). “[Somatostatin and its receptors. Role in the control of cell proliferation]". Minerva Endocrinology
.
26
(3): 91–102. PMID 11753230. - Yamada Y., Rizin T., Lo SF, Ihara Y., Kubota A., Kagimoto S., Seino M., Seino Y., Bell GI, Seino S. (December 1992). "Somatostatin receptors, an expanding gene family: cloning and functional characterization of human SSTR3, an adenylate cyclase-related protein." Molecular endocrinology
.
6
(12): 2136–42. Doi:10.1210/me.6.12.2136. PMID 1337145. - Yamada Y, Post SR, Wang K, Tager HS, Bell GI, Seino S (January 1992). "Cloning and functional characterization of the human and mouse somatostatin receptor family expressed in the brain, gastrointestinal tract, and kidney." Proceedings of the National Academy of Sciences of the United States of America
.
89
(1):251–5. doi:10.1073/pnas.89.1.251. PMC 48214. PMID 1346068. - Brazeau P, Weil V, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R (January 1973). "Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone." The science
.
179
(4068):77–9. Doi:10.1126/science.179.4068.77. PMID 4682131. S2CID 10997771. - Shen LP, Pictet RL, Rutter WJ. (August 1982). "Human somatostatin I: cDNA sequence". Proceedings of the National Academy of Sciences of the United States of America
.
79
(15):4575–9. doi:10.1073/psas.79.15.4575. PMC 346717. PMID 6126875. - Shen LP, Rutter WJ (April 1984). "Sequence of the human somatostatin I gene". The science
.
224
(4645): 168–71. Doi:10.1126/science.6142531. PMID 6142531. - Montminy MR, Goodman RH, Horovitch SJ, Habener JF (June 1984). "Primary structure of the rat preprosomatostatin gene". Proceedings of the National Academy of Sciences of the United States of America
.
81
(11):3337–40. Doi:10.1073/psas.81.11.3337. PMC 345502. PMID 6145156. - Zabel B.W., Naylor S.L., Sakaguchi A.Y., Bell G.I., Shows TB (November 1983). "High-resolution chromosomal localization of human amylase, proopiomelanocortin, somatostatin, and DNA fragment (D3S1) genes by in situ hybridization." Proceedings of the National Academy of Sciences of the United States of America
.
80
(22):6932–6. doi:10.1073/pnas.80.22.6932. PMC 390100. PMID 6196780. - Panetta R., Greenwood M.T., Varshinskaya A., Demchishin L.L., Day R., Niznik H.B., Srikanth S.B., Patel Y.S. (March 1994). "Molecular cloning, functional characterization and chromosomal localization of the human somatostatin receptor (somatostatin receptor type 5) with preferential affinity for somatostatin-28." Molecular Pharmacology
.
45
(3): 417–27. PMID 7908405. - Demchishin L.L., Srikanth S.B., Sunahara R.K., Kent J., Seaman P., Van Tol H.H., Panetta R., Patel Y.K., Niznik H.B. (June 1993). "Cloning and expression of a human somatostatin receptor-14 receptor variant (somatostatin receptor 4) located on chromosome 20." Molecular Pharmacology
.
43
(6):894–901. PMID 8100352. - Kaupmann K., Bruns S., Heuer D., Seuwen K., Luebbert H. (September 1993). "Distribution and second messenger of four somatostatin receptor subtypes expressed in the brain". FEBS Letters
.
331
(1–2):53–9. Doi:10.1016/0014-5793(93)80296-7. PMID 8405411. S2CID 22557713. - Aguila MK, Rodriguez AM, Aguila-Mansilla HN, Lee VT. (May 1996). "Somatostatin antisense oligodeoxynucleotide-mediated stimulation of lymphocyte proliferation in culture". Endocrinology
.
137
(5):1585–90. Doi:10.1210/en.137.5.1585. PMID 8612489. - Sharma K, Patel YK, Srikanth CB (December 1996). "Subtype-selective induction of wild-type p53 and apoptosis but not cell cycle arrest by human somatostatin receptor 3." Molecular endocrinology
.
10
(12): 1688–96. doi:10.1210 / me.10.12.1688. PMID 8961277. - Dournaud P, Boudin H, Schonbrunn A, Tannenbaum GS, Beaudet A (February 1998). "Interaction between sst2A somatostatin receptors and somatostatin-containing axons in the rat brain: evidence for regulation of cell surface receptors by endogenous somatostatin." Journal of Neuroscience
.
18
(3): 1056–71. Doi:10.1523/JNEUROSCI.18-03-01056.1998. PMC 6792775. PMID 9437026. - Barney A, Roberts J, Ho RH. (January 1999). "Evidence for a synergistic effect of the HIV-1 envelope protein gp120 and brain-derived neurotrophic factor (BDNF) leading to enhanced neuronal somatostatin expression in pooled cultures derived from human fetal cortex." Brain Research
.
815
(2):349–57. Doi:10.1016/S0006-8993(98)01098-1. PMID 9878821. S2CID 21793593. - Ferone D, van Hagen PM, van Koetsveld PM, Zuijderwijk J, Mooy DM, Lichtenauer-Kaligis EG, Colao A, Bogers AJ, Lombardi G, Lamberts SW, Hofland LJ (January 1999). "In vitro study of somatostatin receptors in the human thymus and the effects of somatostatin and octreotide on cultured thymic epithelial cells." Endocrinology
.
140
(1):373–80. Doi:10.1210/en.140.1.373. PMID 9886848. - Bracch N., Lazar N., Panchal M., Allemandu F., Boileau G., Cohen P., Rolam M. (February 2002). "Somatostatin-28(1-12)-NPAMAP sequence: an important helical motif driving prosomatostatin processing at mono- and dibasic sites". Biochemistry
.
41
(5):1630–9. Doi:10.1021/bi011928m. PMID 11814357. - Oomen SP, van Hennick PB, Antonissen K, Lichtenauer-Kaligis EG, Hofland LJ, Lamberts SV, Levenberg B, Touw IP. (February 2002). "Somatostatin is a selective chemoattractant for primitive (CD34(+)) hematopoietic progenitor cells." Experimental Hematology
.
30
(2): 116–25. Doi:10.1016/S0301-472X (01) 00772-X. PMID 11823046. - Simonetti M, Dee BC (February 2002). “Structural motifs in the maturation process of peptide hormones. Precursor of somatostatin. I. Conformational study of CD.” Journal of Peptide Science
.
8
(2): 66–79. Doi:10.1002/psc.370. PMID 11860030. S2CID 20438890.
Data are presented on the expression of somatostatin receptors types 1, 2, 3 and 5 in 48 functioning (insulinoma - 45, gastrinoma - 3) and 18 non-functioning neuroendocrine tumors of the pancreas (NET of the pancreas). It was shown that in functioning NETs the expression of type 3 receptors predominated, in non-functioning ones – type 2. Changes in the expression profile of somatostatin receptors types 1, 2 and 3 were noted with an increase in the degree of malignancy of pancreatic NETs.
Somatostatin receptors expression 1, 2, 3, and 5 types in pancreatic neuroendocrine tumors
It was examined the expression of somatostatin receptors 1, 2, 3 and 5 types in 56 pancreatic neuroendocrine tumors: 48 function (45 insulinomas and 3 gastrinomas) and 18 non-function tumors. Twenty of 56 tumors were malignant (6 insulinomas, 9 non-function tumors, 3 gastrinomas). In function tumors dominated expression of receptor of type 3 (48%), and in non-function tumors - type 2 (63.2%). In tumors with metastases the number of cells expressing the receptor type 2 and 3 increased, and receptor type 1 decreased.
Neuroendocrine tumors (NETs) of the pancreas are considered relatively rare neoplasms. At the same time, in recent years there has been a tendency towards an increase in the frequency of their detection, both in connection with the introduction of various methods of radiation diagnostics into clinical practice, and with the improvement of morphological verification. The only method of radical treatment for localized pancreatic NETs is surgery [1, 2]. Unresectable and disseminated NETs require an individual approach to treatment, with recommendations for choosing the optimal method (chemo- or biotherapy) based on the morphological characteristics of the tumor [3].
Currently, synthetic analogs of somatostatin are most often used in the first line of drug therapy for NETs (especially functioning ones) of the pancreas [4, 5, 6, 7]. The antitumor activity of these drugs is due to the suppression of tumor cell proliferation, stimulation of their apoptosis, suppression of their production of growth factors and is based on the binding of specific targets on tumor cells - somatostatin receptors (RS) [8].
In this regard, determining the expression profile of PC in tumor cells is the most important factor in predicting the effectiveness of this treatment method. To date, 5 types of MS are known. For the first time, the expression of all five types of these receptors by pancreatic NET cells was demonstrated in studies by JC Reubi et al. [9], however, recent literature data regarding the immunohistochemical (IHC) determination of various types of MS in pancreatic NETs are contradictory, which served as the basis for this study.
Purpose of the study: to study the features of expression of somatostatin receptors types 1, 2, 3 and 5 in pancreatic NETs of varying degrees of malignancy.
Materials and methods
A histological and IHC study of tumor biopsies (in some cases, biopsies of their metastases) from 56 patients with pancreatic NETs was carried out. In all cases, the diagnosis was verified in the pathological department of MONIKI named after. M.F. Vladimirsky. Patients were examined and/or operated on in the department of surgical endocrinology of MONIKI named after. M.F. Vladimirsky and in the Faculty Surgery Clinic named after. N.N. Burdenko I Moscow State Medical University named after. THEM. Sechenov. For IHC, a wide range of antibody markers (ATs) were used: to chromogranin A, synaptophysin, CD56, proliferating cell nuclei - Ki67; hormones - insulin, gastrin, glucagon, somatostatin, pancreatic polypeptide; somatostatin receptors type 1 (polyclonal, Abbiotic, USA), type 2 (rabbit monoclonal ID-EPR3340, Epitomix, USA), type 3 (polyclonal, Thermoscientific, USA) and type 5 (rabbit monoclonal ID-UMB4, Epitomix, USA) . Analysis of PC expression was carried out in accordance with the method developed by Volante M. et al. [10]. A positive (“++” and “+++”) reaction was considered to be predominantly membrane or membrane-cytoplasmic expression of PC in 50-100% of tumor cells. A weakly positive (“+”) or focal reaction was regarded as negative. The degree of malignancy of pancreatic NETs was determined according to the WHO classification (2010), according to the criteria of which they were divided into highly differentiated NETs of 1 and 2 degrees of malignancy. There were no poorly differentiated grade 3 neuroendocrine carcinomas among the tumors studied.
Results and its discussion
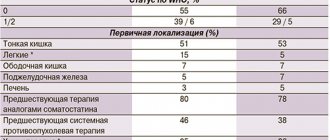
Expression of PC types 1, 2, 3 and 5 was found in all pancreatic NETs studied. In a comparative analysis of the expression of RS types 1, 2, 3 and 5, it was found that in hormonally active and non-functioning pancreatic NETs, the expression of RS-1 was observed in 17.9% and 21.4%, RS-2 – in 41.9 % and 63.2%, RS-3 – in 48% and 25%, RS-5 – in 15.4% and 11%, respectively (Table 1).
Table 1.
Expression of PC in pancreatic NETs depending on their functional activity
| Types of PC | Hormonally active NETs, % | Non-functioning NETs, % |
| RS-1 | 17,9 | 21,4 |
| RS-2 | 41,9 | 63,2 |
| RS-3 | 48,0 | 25,0 |
| RS-5 | 15,4 | 11,0 |
It was also noted that with an increase in the malignant potential of tumors, the expression profile of RS changes (Table 2): in functioning and non-functioning NETs without metastases (grade 1 malignancy), expression of RS-1 was detected in 16.7% and 25% compared to tumors with metastases (grade 2 malignancy) – 10% and 19.4%, respectively.
Table 2.
Expression of PC in pancreatic NETs depending on their malignant potential
| Types of PC | Hormonally active NETs | Non-functioning NETs | ||
| With metastases, % | Without metastases, % | With metastases, % | Without metastases, % | |
| RS-1 | 10,0 | 16,7 | 19,4 | 25,0 |
| RS-2 | 70,0 | 37,1 | 66,7 | 60,0 |
| RS-3 | 66,7 | 47,6 | 33,0 | 20,0 |
| RS-5 | 11,0 | 10,0 | 33,0 | 20,0 |
On the contrary, the number of tumors expressing PC-2 and PC-3 increased. Thus, the expression of PC-2 in hormonally active pancreatic NETs without metastases and with metastases was detected in 37.1% and 70%, respectively, the expression of PC-3 in 47.6% and 66.7%. Among non-functioning NETs without metastases, the number of tumors expressing PC-2 and PC-3 was 60% and 20%, among non-functioning tumors with metastases - 66.7% and 33%. No significant differences in PC-5 expression were detected: in hormonally active pancreatic NETs with and without metastases, PC-5 positive cells were detected in 11% and 10%, respectively, in non-functioning tumors (both with and without metastases ) - at 11%.
The majority of functioning pancreatic NETs were insulinomas, in which expression of RS types 1, 2, 3 and 5 was observed in 17.9%, 41.9%, 48% and 15.4%, respectively (Fig. 1).
Figure 1. RS expression in insulinomas
Metastatic insulinomas expressed these receptors in 12.5%, 62.5%, 50% and 12.5%. The number of insulinomas without metastases expressing RS was 19.4%, 38.2%, 47.6% and 16.1%, respectively (Fig. 2).
Figure 2. Expression of RS in insulinomas of different grades of malignancy
Literature data on IHC determination of the expression of different types of RS in pancreatic NETs are contradictory. This is explained by a number of factors: the absence until recently of sufficiently specific and sensitive antibodies to these receptors, large heterogeneity and a small number of tumors studied, and the lack of a standard method for determining and assessing MS. Currently, such standards have been developed [10, 11]. Moreover, it has been shown that only the membrane, and not the cytoplasmic, type of expression of PC correlates with a positive response to treatment with somatostatin analogues [12]. It is this type of expression that was noted in a study using new, highly specific monoclonal rabbit antibodies [13, 14].
The use of somatostatin analogues for the treatment of unresectable or disseminated pancreatic NETs increases the duration and quality of life of patients. The most important factor in predicting the effectiveness of this therapy is determining the expression profile of RS in tumor cells, and, first of all, determining the expression of RS-2. Along with this, PC-2 expression may also be associated with the malignancy potential of pancreatic NETs, which is confirmed by the results of the study. Thus, with increasing insulin grade, PC-2 expression increased almost 2-fold in tumors with metastases compared to tumors without metastases. On the other hand, the lack of PC-2 expression and the presence of other types of PC may explain the resistance of high-grade insulin to treatment with somatostatin analogues. Our results confirm this fact: almost 50% of insulinomas studied expressed PC-3, and 18% expressed PC-1. Currently, drugs have been developed (in particular, SOM-230) that can bind a wider range of RS, including RS-1 and RS-3, which provides wider possibilities for the treatment of pancreatic NETs [15].
Conclusion
Pancreatic NETs express RS types 1, 2, 3 and 5, with the highest level of expression in functioning tumors being typical for RS-3, and in non-functioning tumors – for RS-2. The increased malignancy potential of insulin-producing pancreatic NETs correlates with increased expression levels of PC-2 in tumor cells. Determining the expression profile of RS in pancreatic NETs makes it possible to individualize their prognosis and optimize treatment tactics through the justified prescription of somatostatin analogues to patients.
L.E. Gurevich, E.I. Panteleeva, N.A. Korsakova, I.A. Kazantseva, A.V. Egorov,
T.A. Britvin, I.A. Vasiliev, E.I. Ustinova
Moscow Regional Research Clinical Institute named after. M.F. Vladimirsky
First Moscow State Medical University named after. THEM. Sechenov
Britvin Timur Albertovich - Doctor of Medical Sciences, leading researcher at the Department of Surgical Endocrinology
Literature:
1. Egorov A.V., Musaev G.Kh., Kondrashin S.A. and others. Factors that determine the immediate results of surgical treatment of insulin-producing pancreatic tumors // Surgery. Journal named after N.I. Pirogov. - 2011. - No. 6. - P. 1-6.
2. Britvin T.A., Gurevich L.E., Dreval A.V. and others. Diagnosis and treatment of neuroendocrine tumors of the pancreas. A manual for doctors. - M.: Linko Group, 2012. - 23 p.
3. Gurevich L.E. Neuroendocrine tumors of the gastrointestinal tract and pancreas // Book. "Guide to the immunohistochemical diagnosis of human tumors", 4th edition, expanded and revised. - 2012. - Ch. 34. - pp. 469-489.
4. Gorbunova V.A., Orel N.F., Kuzminov A.E. Modern directions in the treatment of neuroendocrine tumors //Modern Oncology. - 2010. - No. 1. - P. 7-12.
5. Guillermet-Guibert J., Lahlou H., Pyronnet S. et al. Somatostatin receptors as tools for diagnosis and therapy: molecular aspects // Best Practice and Research Clinical Gastroenterology. - 2012. - Vol. 1, No. 4. - P. 535-551.
6. Oberg KE, Reubi JC, Kwekkeboom DJ, Krenning EP Role of somatostatins in gastroentrohancreatic neuroendocrine tumor development and therapy // Gastroenterology. - 2010. - Vol. 139, No. 3. - P. 742-75.
7. Jais P., Terris B., Ruszniewski P. et al. Somatostatin receptor subtype gene expression in human endocrine gastroentero-pancreatic tumors // Eur. J. Clin. Invest. - 1997. - Vol. 27, No. 8. - P. 639-64.
8. Strosberg J., Kvols L. Antiproliferative effect of somatostatin analogs in gastroenteropancreatic neuroendocrine tumors // World J. Gastroenterol. - 2010. - Vol. 16, No. 24. - P. 2963-2970.
9. Reubi JC Somatostatin receptors in the gastrointestinal tract in health and disease // Yale Journal of biology and medicine. - 1992. - Vol. 65, No. 5. - P. 493-536.
10. Volante M., Brizzi M., Faggiano A. et al. Somatostatin receptor type 2a immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy // Mod. Pathol. - 2007. - Vol. 20. - P. 1172-1182.
11. Papotti M., Bongiovanni M., Volante M. et al. Expression of somatostatin receptor types 1-5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. A correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis // Virchows Arch. - 2002. - Vol. 440, No. 5. - P. 461-475.
12. Bertherat J., Tenenbaum F., Perlemoine K. et al. Somatostatin receptors 2 and 5 are the major somatostatin receptors in insulinomas: an in vivo and in vitro study // J. Clin. Endocrinol. Metab. - 2003. - Vol. 88. - P. 5353-5360.
13. Korner M., Waser B., Schonbrunn A. et al. Somatostatin receptor subtype 2a immunohistochemistry using a new monoclonal antibody selects tumors suitable for in vivo somatostatin receptor targeting // Am. J. Surg. Pathol. - 2012. - Vol. 36. - P. 242-252.
14. Schmida HA, Lambertinib C, van Vugta HH et al. Monoclonal antibodies against the human somatostatin receptor subtypes 1-5: development and immunohistochemical application in neuroendocrine tumors // Neuroendocrinol. - 2012. - Vol. 95. - P. 232-247.
15. Appetecchia M., Baldelli R. Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumors, current aspects and new perspectives // J. Exp. Clin. Canc. Res. - 2010. - Vol. 29, No. 1. - P. 19-31