Everolimus (Afinitor) is an mTOR inhibitor. The PI3K/AKT/mTOR intracellular signaling pathway, which regulates the survival, growth and proliferation, metabolism and angiogenesis of tumor cells, is one of the main ones in tumor development or carcinogenesis. In addition, activation of the mTOR pathway is observed in genetic cancer (von Hippel–Lindau disease, neurofibromatosis type I) and other (tuberous sclerosis) syndromes.
Intracellular interactions are extremely diverse. However, some mechanisms of these interactions between different target organs are already known and, moreover, are used in clinical practice for the combination of targeted drugs.
An example of such combinations is the use of everolimus in combination with hormone therapy (HT) to restore sensitivity to the latter in disseminated breast cancer (BC) or with octreotide in neuroendocrine tumors (NET).
Based on its mechanism of action, everolimus has the potential to be effective in a number of tumors; The first indication for its clinical use was renal cell carcinoma (RCC). The drug is included in clinical recommendations as a 2nd line of therapy for disease progression after therapy with tyrosine kinase inhibitors (sunitinib, sorafenib, pazopanib).
Everolimus has been most fully studied in NETs, in which three randomized clinical trials RADIANT were conducted.
A preliminary phase II study showed the effectiveness of the combination of everolimus with octreotide in pancreatic NETs (PG), and in the first randomized comparative trial (RADIANT-1) the combination was found to be more effective than everolimus alone: control of tumor growth - 84.4 versus 77%, median progression-free survival (PFS) – 16.7 versus 9.7 months [1].
Two more studies were conducted in Phase III. One of these is RADIANT-2, a double-blind placebo-controlled trial comparing everolimus + octreotide (E + O) with placebo plus octreotide (R + O) for patients with secreting tumors. The characteristics of the patients are presented in Table. 1.
The main objective of the study was to evaluate PFS. According to central analysis, the median was higher in the everolimus group (16.4 versus 11.3 months; p = 0.026). The threshold p value for the study design should have been 0.0246. According to the researchers, the results were somewhat different: MTFS E + O – 12.0 months, P + O – 8.6 months (p = 0.018).
In this regard, an independent committee reviewed the results again and came to the final conclusion that there was a significant improvement in FSFS by 5.1 months when treated with the E + O combination in patients with NETs with secretory activity (Table 2) [2].
The study confirmed this benefit in a subgroup analysis and also showed improved control of 5-hydroxyindoleacetic acid levels, which is a prerequisite for preventing the development of carcinoid heart disease.
The lung NET subgroup included 44 out of 429 patients, i.e. approximately 10%. The MTFS was 13.6 versus 5.5 months in favor of the E + O combination. The risk ratio (HR) was 0.72. In this regard, this combination is registered as a treatment for patients with NET of the pancreas and lungs in the Russian Federation.
The RADIANT-3 study compared the best treatment for patients with low-grade or moderate-grade NET (Gr1 and Gr2), supportive care (NT), + everolimus, and NT + placebo. The MTFS for everolimus was 11 months, for placebo – 4.6 (p < 0.0001). Treatment time was 8.79 months in the everolimus group and 3.70 in the placebo group; with progression, 148 patients in the placebo group switched to the everolimus group. At the same time, the 18-month survival rate in the everolimus group was 57.3%, and the two-year survival rate was 34.2%. The most common adverse events with everolimus were stomatitis (64%), rash (49%), diarrhea (34%), fatigue (31%), and infections (23%).
Thus, the RADIANT research program, which included 999 patients, showed the effectiveness of everolimus in monotherapy of pancreatic NETs and in combination with octreotide for secreting NETs.
In the chemotherapy department of the Federal State Budgetary Institution "Russian Cancer Research Center named after. N.N. Blokhin" of the Russian Academy of Medical Sciences has experience in using everolimus in 21 patients, 11 of whom had pancreatic NETs. 11 patients suffered from Gr2 tumors, 18 from liver metastases. Previously, 9 patients underwent cytoreductive surgery, 19 patients received everolimus as 2nd line of therapy. A partial effect was noted in 1 (5%) patient, stabilization – in 14 (67%). Thus, control of tumor growth was achieved in 72% of cases. The MTFS was 4.3 months. We noted that the best long-term results occurred in the group of patients to whom everolimus was used as the 1st or 2nd line of treatment: MTFS - 6.9 versus 3.4 months with large values of the number of lines.
The third tumor for which everolimus is approved for clinical use is breast cancer. The most common mutations in breast cancer occur in the PI3K/AKT/mTOR signaling pathway [3].
Everolimus has been studied in hormone-sensitive breast cancer resistant to previous hormonal therapy. Such resistance occurs in 25% of patients with the presence of estrogen receptors (ER+) and progesterone and in 50% of patients with other characteristics.
Approximately 50% of patients with ER+ metastatic breast cancer (mBC) fail to respond to first-line hormonal therapy, indicating primary resistance. Ultimately, the remaining patients progress despite the initial response. This process is called acquired resistance.
Growth factor signaling activity is considered an important component of resistance. Cross-activation may occur between the ER, IGFR, and EGFR/HER2 signaling pathways. Activation of membrane receptors of these growth factors can lead to stimulation of two main signaling cascades of intracellular kinases: PI3K/AKT/mTOR and RAS/MAPK. Preclinical studies have demonstrated cross-activity between the ER and mTOR signaling pathways (Fig. 1).
Treatment options that combine hormonal and targeted agents may improve the effectiveness of therapy and prevent or delay the onset of endocrine resistance in breast cancer.
Randomized clinical trials of everolimus for ER+ breast cancer included phase II (letrozole ± E and tamoxifen ± E) and phase III studies: BOLERO–2 (exemestane ± E).
A phase II study showed superiority of neoadjuvant letrozole + E (n = 138) compared with letrozole plus placebo (n = 132) in terms of objective response (ER): 58 vs. 47%; p = 0.035 (assessed by ultrasound). A reduction in the expression of Ki-67 (a cell proliferation marker) was observed among 57% of patients receiving everolimus, and only among 30% of the control group [5].
In the BOLERO-2 study, the primary goal was achieved - a statistically significant improvement in FSFS to 6.9 months compared with 2.8 in the placebo + exemestane group (HR = 0.45; p < 0.0001). A total of 724 patients were included in the study, incl. there were 485 people in the group receiving everolimus 10 mg/day and exemestane 25 mg/day (group E + E), and 239 in the placebo + exemestane group. All patients were postmenopausal and had ER+-, HER2-locally advanced or metastatic process with relapse or progression after the use of letrozole or anastrozole.
In 84% of all patients, tumors were hormone sensitive.
PFS analysis was performed at 18-month follow-up, showing an increase in FSFS for the everolimus group of 4.6 months (7.8 vs 3.2; HR = 0.45; p < 0.0001).
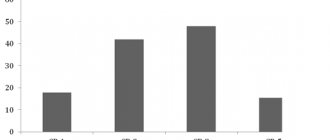
The advantage of everolimus remained in separate analysis in all subgroups of patients. Improvement was achieved in the presence of visceral metastases, without them, and in patients with only bone metastases (Fig. 2) [6].
Analysis of the results of a group of patients who had previously received chemotherapy also showed a significant advantage of the E + E regimen: MTFS was 7.1 versus 2.2 months when using P + E.
The secondary objectives of the BOLERO-2 study were to evaluate overall survival (OS), OS, clinical efficacy (OS + stable disease), quality of life, safety and pharmacokinetics.
Assessment of quality of life demonstrated a more than twofold increase in MVBP in the E + E group with good physical, emotional and social status of patients; the time for maintaining physical activity was 15.2 months in the E + E group and 9.7 in the P + E group (p = 0.0211).
OS in the BOLERO-2 study was assessed at 7, 12.5 and 18 months [7–9]. In the E + E and P + E groups, it was 89.3 versus 87.0, respectively, at these times; 82.7 versus 77.3 and 74.6 versus 67.8%. With a median follow-up duration (October 03, 2013), in the E + E group it was 44.9%, and in the P + E group – 40.2%. Another 13 patients remained under treatment.
Median OS was 30.98 months for the E + E group and 26.55 for the P + E group (HR = 0.89; p = 0.14) [10].
When analyzing subsequent treatment, it turned out that in the P + E group there were 10% more patients receiving chemotherapy, incl. vinorelbine and taxanes.
The median time from randomization to first chemotherapy or death was 11.86 months in the E + E group and 5.98 in the P + E group. No new toxicities were noted [10].
In summary, the BOLERO-2 study did not find significant differences in OS between the E+E and P+E groups. However, given the clear trend toward increased OS in the E+E group, subgroup analyzes of these results may be warranted.
In 2014, the results of a selective analysis of FSFS in a subgroup of patients who had previously received letrozole or anastrozole in the neoadjuvant setting were published [11]. In this subgroup of patients with HR+-, HER-BC, the combination of E + E caused an almost threefold increase in FSFS compared with P + E according to local assessment (11.5 versus 4.1 months), which was confirmed by centralized assessment (15.5 versus 4.2 months).
It is important to note that a good quality of life is maintained with the E+E combination despite the side effects of everolimus. Time to definitive deterioration (TDD) according to global health assessment was noticeably longer in the everolimus group (11.1 versus 7.2 months; Fig. 3) [11].
If we compare the results obtained with the capabilities of any other HT after the use of non-steroidal aromatase inhibitors, the results of its use were less impressive: 3.7 months - fulvestrant + exemestane (EFECT); 4.4 – fulvestrant 250 mg + anastrozole, 4.8 – fulvestrant 250 mg and 3.4 months – exemestane (SOFEA); 6.5 months – fulvestrant 500 mg and 5.5 – fulvestrant 250 mg (CONFIRM).
Studies of HT in breast cancer in combination with targeted agents are still quite limited. In the HORIZON study, temsirolimus plus letrozole did not produce the expected adequate improvement compared with letrozole alone, although 56% of patients in this study had not received prior ADT.
Thus, the results of the above selective analysis of data from the BOLERO-2 study serve as a prerequisite for the use of the E + E combination as the 1st line of treatment for patients after neoadjuvant/adjuvant therapy with non-steroidal aromatase inhibitors.
The BOLERO-4 trial is currently underway, examining the combination of everolimus with letrozole as first-line therapy in HR+-, HER-mBC. A protocol has also been initiated to add everolimus to adjuvant hormonal therapy.
Also, based on BOLERO-2 data, a study of exon sequences and gene copy number variants was carried out using Next-Generation Sequencing (NGS), which was carried out using tumor samples from 227 of 724 patients. 182 tumor-related genes were studied. A therapeutic benefit of E+E greater than that of exemestane alone was confirmed for each of the 9 genes with mutation rates <10% (eg, PIK3CA, FGFR1, CCND). 76% of patients with minimal genetic alterations in the PI3K, FGFR, or CCND signaling pathways were found to benefit most from everolimus (RR = 0.27 vs. 0.40 for the full NGS population). This approach may be popular in the future [12].
Another direction for studying everolimus in mBC is its use in the event of resistance to trastuzumab. From the point of view of the mechanism of action of the drug, there are theoretical prerequisites for the effectiveness of everolimus in this situation (Fig. 4) [13].
The BOLERO-3 study compared everolimus 5 mg daily in combination with vinorelbine (B) 25 mg/m2/week and trastuzumab (T) 2 mg/kg/week with placebo + B + T in locally advanced or previously treated mBC. T (100%), taxanes (100%), lapatinib (28% of patients). Visceral metastases were found in 76% of cases. The primary objective of the study was to evaluate PFS: FSFS for E + V + T was 7 months, for P + V + T = 5.78 (HR = 0.78; p = 0.0067).
In conclusion, E+B+T is an effective combination that significantly prolongs PFS in mBC with a 22% reduction in the risk of progression or death, but it is not yet approved. Apparently, additional clinical studies and a search for the most optimal characteristics of tumors from the point of view of achieving a significant gain in effect are necessary. In the meantime, groups have been identified in which the addition of everolimus gave the least result. These are patients with liver damage (RR = 0.90; 95% CI – 0.68–1.18), patients who have never received adjuvant trastuzumab (RR = 0.92; 95% CI – 0.71–1.18 ), and ER+ (RR = 0.94; 95% CI 0.72–1.23).
When assessing the OE, no additional significant benefit was noted from the addition of everolimus: 40.8 versus 37.3%; tumor growth control (TE + stabilization) – 59.2 versus 53.3%.
Improved PFS was reported among patients with minimal changes in PI3K, PTEN, CCND1, or FGFR1/2 (Figure 5).
This additional study highlights the importance of the involvement of two or more key signaling pathways in carcinogenesis and their possible interactions.
In the TAMRAD study (tamoxifen + everolimus versus tamoxifen; n = 111), tumor marker analysis performed on 51 patients with advanced HR+ breast cancer showed that the effectiveness of everolimus was higher with low expression of PI3K and the LKB1 antioncogene, a tumor suppressor gene of the hepatic B1/serine/threonine kinases. The greatest effectiveness was observed with high expression of the p4EBP1 protein, which characterizes downstream effector mechanisms. Thus, activation of the mTOR signaling pathway is the best predictor of everolimus efficacy. This condition is characterized by low expression of the PIK3CA gene signature, low LKB1 and high p4EBP1.
The main side effects with everolimus are stomatitis, fatigue, rash, hyperglycemia, and infections. A comparison of the specific toxicity of the drug when used in doses of 5 (BOLERO-3) and 10 mg/day (BOLERO-2) orally is presented in Table. 3.
Stomatitis was more severe in BOLERO-3, most likely due to the combination of everolimus with vinorelbine chemotherapy.
The occurrence of hypertriglyceridemia, noted in many patients receiving everolimus in RECORD-1 (71% in RCC) and a number of other studies, has also been described (Table 4) [14–17].
There are rare descriptions of the occurrence of acute pancreatitis during the use of everolimus [18]. Skin rash, according to a meta-analysis that included 2242 patients from 13 clinical studies, occurred in 28.6% of patients receiving the drug, incl. in 1% it is severe [19]. Patients receiving everolimus are also characterized by the development of mucositis, anemia, and nausea, but the incidence of severe cases of these effects is insignificant [20]. Taking into account possible infectious complications, the drug should be prescribed with caution to elderly and debilitated patients.
Thus, everolimus is an inhibitor of the serine-threonine kinase region of the PI3K/AKT signaling pathway, which is critical for cell growth and angiogenesis. The drug binds intracellular protein, which leads to inhibition of mTOR kinase activity. Additionally, it inhibits the expression of hypoxia-inducing factor and reduces the expression of epidermal growth factor (EGFR).
Everolimus was initially approved by the US Food and Drug Administration (FDA) in 2009 as a second-line treatment for advanced RCC. It was then approved for the treatment of subependymal giant cell astrocytomas associated with tuberous sclerosis (2010), progressive pancreatic NETs (2011), and renal angiolipomyoma as part of a complex of lesions in tuberous sclerosis.
The latest indication for the use of everolimus was HR+-, HER2-mBC, previously treated with non-steroidal aromatase inhibitors (2012). Everolimus has also shown promising results in kidney and heart transplantation [21, 22].
Detailed description of the study
Everolimus (active ingredient Everolimus, trade names "Afinitor", "Certican", "Nicolimus") is a drug with antitumor activity that selectively suppresses the immune system. It is a derivative of sirolimus and prevents the intensive proliferation of T-lymphocytes.
In the body, T-helpers, T-suppressors and NK (natural killer) cells are responsible for recognizing foreign microorganisms (antigens) and destroying them. They regulate the strength of the immune response and produce cytokines - low molecular weight peptides that distribute a signal between the cells of the immune system.
Normally, T-lymphocytes ensure the integrity of the immune system, prevent the development of bacterial and viral infections, and malignant degeneration of cells. In patients who have undergone an organ transplant, stimulation of T-lymphocytes can provoke a rejection reaction, because transplanted tissues are recognized as foreign. To maximize the chances of engraftment after transplantation, immunosuppressants are prescribed.
A special feature of the medicine is its selectivity of action, which distinguishes it from other representatives of this group. By suppressing the T-cell component of immunity, it does not inhibit the activity of other components of the immune system, preserving the body’s ability to protect itself from viruses and bacteria.
In addition to transplantation, the drug is quite widely used in the treatment of certain oncological diseases. Its demand is associated with the ability to inhibit, up to a complete stop, active cellular growth, characteristic of malignant neoplasms. Control of tumor spread, including metastasis, is observed (in some pathologies) in 7 out of 10 patients.
Everolimus dosage regimens involve in most cases a combination with basiliximab, cyclosporine and glucocorticosteroids. At the same time, a change in pharmacotherapy, the exclusion of a particular drug from it, often provokes a change in the concentration of the active substance in the blood plasma. Such fluctuations can significantly reduce the effectiveness of conservative treatment. In such situations, a blood test for the concentration of everolimus helps to avoid the development of adverse reactions associated with a decrease in drug clearance. The need for dosage adjustment may also arise when switching from one dosage form to another (dispersible tablets, regular tablets).
The optimal therapeutic concentration of the drug is from 3 to 8 ng in one milliliter of blood. When this indicator decreases, the risk of rejection increases significantly; when it increases, the risk of unwanted side reactions develops:
- Dose-dependent decrease in the number of leukocytes, platelets, red blood cells;
- Disturbances in the functioning of the coagulation system;
- Increased levels of triglycerides, low-density lipoproteins;
- Arterial hypertension;
- Pneumonitis;
- Attachment of secondary bacterial and fungal infections of the urinary tract, respiratory system, skin;
- Liver dysfunction: liver failure, hepatitis, transient hyperbilirubinemia, increased transaminase levels.
The drug has a narrow therapeutic window (the range between therapeutic and toxic doses), so its level must be monitored.
References
- Bezvulyak, E.I., Vavilova, T.V., Basharin, V.A. and others. Pharmacological and laboratory aspects of therapeutic drug monitoring of everolimus. - Medline.Ru., 2021. - T. 19(29). — P. 392-406.
- Bezvulyak, E.I., Melnichenikova, O.S., Vavilova, T.V. et al. Optimization of monitoring of everolimus concentrations in patients after heart transplantation against the background of liver pathology. - Experimental and clinical gastroenterology, 2021. - T. 150 (2). — P. 68-73.
- Campistol, D., Haldaas, H. Everolimus and long-term results of kidney transplantation. - Journal of Transplantation, 2011. - T. 92. - No. 3S. — P. 303-310.
- Bilbao, I., Dopazo, C., Lazaro, J. et al. Multiple indications for everolimus after liver transplantation in current clinical practice. - World J. Transplant., 2014. - Vol. 4(2). — P. 122–132.
- Martins, F., de Oliveira, M., Qian Wang, Q. et al. A review of oral toxicity associated with mTOR inhibitor therapy in cancer patients. - OralOncol., 2013. - Vol. 49(4). — P. 293–98.
- Formicajra, R., Lorberb, K., Friedmanb, A. et al. The evolving experience using everolimus in clinical transplantation. - Elsevier: journal, 2004. - Vol. 36(2). — P. S495-S499.