Pharmacological properties of the drug Finasteride
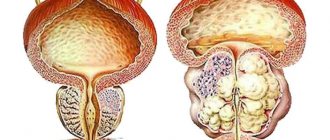
A synthetic 4-azasteroid compound, a specific inhibitor of 5-α-reductase, an intracellular enzyme that converts testosterone into a more active androgen - dihydrotestosterone (DHT). In benign prostatic hyperplasia (BPH), its increase is caused by the conversion of testosterone to DHT in the prostate tissue. Finasteride is a highly effective drug that reduces the level of DHT in the blood and prostate gland. Finasteride has no affinity for androgen receptors. Finasteride treatment causes regression of prostatic hypertrophy, steadily increases the maximum urinary flow rate and improves clinical symptoms.
Finasteride (Propecia/Proscar)
Testosterone in men is produced primarily in the testicles and also in the adrenal glands. Most testosterone in the body is bound to sex hormone binding globulin (SHBG), a protein made in the liver that transports testosterone through the blood, prevents its metabolism, and prolongs its half-life. When freed from SHBG, free testosterone can enter cells throughout the body. In some tissues, particularly the scalp, skin and prostate, testosterone is converted to 5alpha-dihydrotestosterone (DHT) by the enzyme 5-alpha reductase. DHT is a more potent androgen than testosterone (with approximately 3-10 times more activity at the androgen receptor, the site of action of androgen hormones), so 5alpha reductase may be considered to enhance the androgenic effects of testosterone in the tissues where it is found. Finasteride, a 4-azasteroid and testosterone analogue, acts as a potent and specific, competitive inhibitor of one of the two subtypes of 5alpha reductase, in particular the type II isoenzyme. In other words, it binds to the enzyme and prevents the metabolism of endogenous substrates such as testosterone. 5α-reductases type I and II account for approximately one-third and two-thirds of systemic DHT production, respectively. Other 5a-reductase substrates include progesterone, androstenedione, epi-testosterone, cortisol, aldosterone, and deoxycorticosterone. The full physiological effect of their restoration is unknown, but it is likely related to their release or is itself physiological. In addition to acting as a catalyst in the rate-limiting testosterone reduction reaction, isoforms I and II of the 5alpha reductase enzyme reduce progesterone to dihydroprogesterone (DHP) and deoxycorticosterone to dihydrodeoxycorticostenor (DHDC). In vitro and animal models show that subsequent 3alpha reduction of DHT, DHP and DHDOC results in the creation of steroid metabolites that affect brain function by increasing GABA inhibition of gamma-aminobutyric acid. These neuroactive steroid derivatives increase GABA levels at GABA(A) receptors and have anticonvulsant, antidepressant, and anxiolytic effects, as well as influencing sexual and alcohol-related behavior. 5alpha-dihydrocortisol from intraocular fluid is synthesized in the lenses, and can itself participate in the production of intraocular fluid. Allopregnanolone and DGDOK are neurosteroids, and the latter may influence the susceptibility of animals to epilepsy. 5alpha-dihydroaldosterone is a potent antidiuretic, although it is different from aldosterone. Its formation in the kidneys is increased by limiting dietary salt, suggesting that sodium can be conserved by:
Substrate + NADPH + H + → 5alpha substrate + NADP +
5alpha-DHP is one of the main hormones in the bloodstream of women with normal cycles and pregnant women. By inhibiting 5-alpha reductase, Finasteride prevents the formation of testosterone in DHT by the type II isoenzyme, resulting in a decrease in serum DHT levels by approximately 65-70%, and an increase in DHT levels in the prostate to 85-90%, where it is dominant. expression of type II isoenzyme. Unlike dual inhibitors of both 5alpha-reductase isoenzymes, which can reduce DHT levels throughout the body by more than 99%, Finasteride is not able to completely suppress the production of DHT, since it cannot have a significant inhibitory effect on the type I 5alpha-reductase isoenzyme, having 100 times lower affinity for I compared to II. In addition to blocking the type II isoenzyme, Finasteride competitively inhibits the type II 5beta-reductase isoenzyme, but this is not thought to affect androgen metabolism. By blocking the production of DHT, Finasteride reduces androgen activity in the scalp. In the prostate, inhibition of 5alpha reductase reduces prostate volume, reducing the development of benign prostatic hyperplasia (BPH) and the risk of prostate cancer. Inhibition of 5alpha reductase also reduces epididymal weight, reduces motility, and alters the normal positioning of sperm within the epididymis. Cause of Mood-Related and Sexual Side Effects DHT and neuroactive steroids (NAS), such as allopregnanolone (ALLO) and tetrahydrodeoxyorthicosterone (THDOC), are potent positive allosteric modulators of the GABA receptor (affecting the same sites as euphoria-inducing substances and anxiolytic drugs such as benzodiazepines and alcohol) and are important endogenous neuroregulators with potent antidepressant and anxiolytic effects, as well as playing a positive role in sexual functioning. Their biosynthesis depends on both isoforms of 5alpha reductase. Finasteride reduces their formation in the body. This effect of Finasteride is the likely cause of the emotional and sexual side effects associated with the drug. Additionally, since it involves not only DHT but also NAS, it could potentially also explain the fact that mood and anxiety-related effects are seen in women as well as men.
Special instructions for the use of Finasteride
With a large volume of residual urine and/or with a sharply reduced urine outflow, it is necessary to strictly monitor the patient’s condition, as obstructive uropathy may develop. So far, no clinically beneficial effect has been found from treating prostate cancer patients with finasteride. Patients with BPH and elevated PSA levels were followed in controlled clinical trials with serial PSA testing and prostate biopsy. In these studies, finasteride did not affect the detection rate of prostate cancer. The overall incidence of prostate cancer was not significantly different between patients receiving finasteride or placebo. Before starting and periodically during treatment with finasteride, it is recommended to conduct a digital rectal examination, as well as other types of studies to exclude the presence of prostate cancer. PSA level determination is also used in medical practice to detect prostate cancer. As a rule, a PSA level above 10 ng/ml indicates the need for further laboratory tests and, possibly, a biopsy; Further laboratory tests are also indicated at PSA levels of 4–10 ng/ml. There was significant similarity in PSA levels between men with and without diagnosed prostate cancer. Therefore, in men with BPH, PSA levels in the normal range do not exclude the presence of prostate cancer, regardless of finasteride treatment. A PSA level exceeding 4 ng/ml does not exclude the presence of prostate cancer. Finasteride reduces PSA concentrations by approximately 50% in patients with BPH, even in the presence of prostate cancer. It should be taken into account that in patients with BPH taking finasteride, a reduced serum PSA level does not exclude the simultaneous presence of a malignant tumor. This decline in PSA levels can be predicted with reasonable confidence over a fairly wide range of values, although it may vary in individual cases. According to clinical studies, when determining PSA in patients taking finasteride, the values obtained should be doubled compared to normal values in men. This correction allows us to maintain the sensitivity and specificity of the method and its diagnostic value for detecting prostate cancer. If there is a persistent increase in PSA levels in patients who have previously been prescribed finasteride, this indicator should be carefully analyzed, without excluding the possibility that the patient did not take finasteride. The concentration of PSA in the blood plasma depends on the age of the patient and the size of the prostate gland; in turn, the volume of the prostate gland depends on the age of the patient. When interpreting the results of PSA laboratory tests, it should be taken into account that, as a rule, PSA levels are usually reduced in patients taking finasteride. In most patients, a rapid decrease in PSA levels is observed already during the first months of treatment, after which the PSA level stabilizes to a new initial value, which is 2 times less than that before treatment. Thus, when treated with finasteride for 6 months or more, the PSA level should be doubled to compare with normal values in men. Due to the ability of 5-α-reductase inhibitors to inhibit the conversion of testosterone to DHT, such drugs (including finasteride) may cause disturbances in the development of the external genitalia of the male fetus and should therefore be avoided by pregnant women or women who may become pregnant. finasteride. Not intended for use in pediatrics.
Finasteride-MIC
General measures
It is necessary to strictly monitor the possible development of obstructive uropathy in patients with a large residual urine volume and/or a sharply reduced urine outflow.
Impact on prostate-specific antigen (PSA) and prostate cancer diagnosis
To date, no beneficial clinical effect of treatment with finasteride in patients with prostate cancer has been identified. Patients with prostate adenoma and elevated PSA levels were followed in controlled clinical trials with multiple PSA determinations and prostate biopsies. In these studies, treatment with finasteride did not affect the incidence of prostate cancer. The overall incidence of prostate cancer did not differ significantly between the groups of patients who received finasteride or placebo.
Before starting treatment and periodically during treatment with finasteride, it is recommended to screen patients by rectal examination, as well as by other methods, for the presence of prostate cancer. Serum PSA testing is also used to detect prostate cancer. In general, if the initial PSA level is greater than 10 ng/ml (Hybritech), the patient should be carefully evaluated, including, if necessary, a biopsy. If the PSA level is between 4-10 ng/ml, further examination of the patient is recommended. Men with and without prostate cancer may have similar PSA levels. Thus, in men with prostate adenoma, a normal PSA value does not rule out prostate cancer, regardless of treatment with finasteride. A baseline PSA level below 4 ng/ml does not exclude the presence of prostate cancer.
Finasteride causes a decrease in serum PSA levels of approximately 50% in patients with prostate adenoma, even in the presence of prostate cancer. This decrease in serum PSA levels in patients with prostate adenoma who are treated with finasteride must be taken into account when assessing PSA levels, since this decrease does not exclude concomitant prostate cancer. This decline is expected across the entire range of PSA levels, although it may fluctuate in some patients. Analysis of PSA data confirmed that typical patients who receive the drug for 6 months or more should have PSA values doubled compared to normal values in individuals who are not receiving treatment. This adjustment preserves the sensitivity and specificity of PSA testing and maintains its ability to detect prostate cancer. Any prolonged increase in PSA levels in a patient receiving treatment with finasteride requires careful evaluation to determine the cause, including non-compliance with the drug.
Finasteride does not significantly reduce the percentage of free PSA (the ratio of free PSA to total PSA). The ratio of free to total PSA remains constant even under the influence of finasteride. When determining the percentage of free PSA, which is used to diagnose prostate cancer, correction of its values is not necessary.
Breast cancer has been reported in men taking finasteride 5 mg. Patients should inform the doctor about any changes in the breast tissue (thickness, pain, gynecomastia, nipple changes), liver failure (the effect on the pharmacokinetics of finasteride has not been studied).
There is insufficient experience with finasteride in patients with hepatic impairment.
The drug contains lactose. The drug should not be used in patients with rare hereditary disorders such as galactose intolerance, congenital lactase deficiency or glucose-galactose malabsorption syndrome.
Taking finasteride may increase the risk of high-grade prostate cancer.
According to the results of clinical studies of the drug Proscar (finasteride) (Prostate Cancer Prevention Trial (PCRT)) when taking finasteride 5 mg per day for 7 years in men 55 years of age and older with normal prostate size (by digital rectal examination) and PSA ≤3.0 ng/ml increased the incidence of well-differentiated prostate cancer (Gleason score 8-10) compared with placebo.
Drug interactions Finasteride
Clinically significant interactions with other drugs have not been noted. Finasteride does not appear to have a significant effect on the cytochrome P450-related enzyme system involved in the metabolism of certain drugs. Clinical trials have examined combinations with propranolol, digoxin, glyburide, warfarin, theophylline and antipyrine. In clinical studies, finasteride has also been used in combination with ACE inhibitors, α- and β-adrenergic blockers, calcium channel blockers, nitrates, diuretics, H2-histamine receptor antagonists, HMG-CoA reductase inhibitors, NSAIDs, quinolones and benzodiazepines. However, no clinically significant negative reactions were found.
Finasteride-obl
Manufacturer: FP OBOLENSKOYE CJSC (Russia)
tab., cover coated, 5 mg: 7, 10, 14, 20, 21, 28, 30, 40, 42 or 56 pcs. Reg. No.: LS-002040
Clinical and pharmacological group:
A drug for the treatment of benign prostatic hyperplasia. 5α-reductase inhibitor
Release form, composition and packaging
| Film-coated tablets | 1 tab. |
| finasteride | 5 mg |
10 pieces. — cellular contour packages (1) — cardboard packs. 10 pieces. — contour cell packaging (2) — cardboard packs. 10 pieces. — cellular contour packages (3) — cardboard packs. 10 pieces. — contour cell packaging (4) — cardboard packs. 14 pcs. — cellular contour packages (1) — cardboard packs. 14 pcs. — contour cell packaging (2) — cardboard packs. 14 pcs. — cellular contour packages (3) — cardboard packs. 14 pcs. — contour cell packaging (4) — cardboard packs. 7 pcs. — contour cell packaging (4) — cardboard packs. 7 pcs. — cellular contour packages (1) — cardboard packs. 7 pcs. — contour cell packaging (2) — cardboard packs. 7 pcs. — cellular contour packages (3) — cardboard packs.
Description of the active components of the drug "Finasteride"
pharmachologic effect
An inhibitor of 5-alpha reductase, an enzyme that converts testosterone into the more active dihydrotestosterone. Reduces the content of dihydrotestosterone in the blood and prostate tissue. Inhibits the stimulating effect of dihydrotestosterone on the development of prostate adenoma.
Finasteride helps reduce the size of an enlarged prostate, improves urine flow and reduces symptoms associated with benign prostatic hypertrophy. Several months of treatment may be required to reduce the clinical manifestations of the disease.
Indications
Benign prostatic hyperplasia (to reduce the size of the prostate; improve urinary flow and reduce symptoms associated with hyperplasia; reduce the risk of acute urinary retention requiring catheterization or surgery, including transurethral resection of the prostate and prostatectomy).
Dosage regimen
The daily dose is 5 mg, the frequency of administration is 1 time/day. The treatment is long-term.
Side effect
From the reproductive system:
rarely - impotence, decreased libido, decreased ejaculate volume, gynecomastia.
Allergic reactions:
skin rash and angioedema are possible.
Contraindications
Hypersensitivity to finasteride, obstructive uropathy, prostate cancer. Finasteride is not used in women and children.
Pregnancy and lactation
Women of childbearing age and pregnant women should avoid contact with the drug, because it has teratogenic properties (the ability to suppress the conversion of testosterone to dihydrotestosterone can cause disruption of the development of genital organs in a male fetus), penetrates into the seminal fluid.
Use for liver dysfunction
Finasteride is prescribed with caution in case of liver failure.
Application for children
The drug is not used in children.
special instructions
Finasteride is prescribed with caution in case of liver failure.
With a large volume of residual urine and/or a sharply reduced urine flow, it is necessary to keep in mind the development of obstructive uropathy.
Before starting treatment with finasteride and periodically during treatment, a rectal examination should be performed, as well as examination by other methods for the presence of prostate cancer.
Drug interactions
No clinically significant interactions were found between finasteride and propranolol, digoxin, glyburide, warfarin, theophylline and antipyrine.
Apparently, finasteride does not have a significant effect on the cytochrome P450 enzyme system and, accordingly, does not affect the pharmacokinetic parameters of drugs metabolized by microsomal liver enzymes.
With simultaneous use of finasteride with ACE inhibitors, alpha-blockers, beta-blockers, calcium channel blockers, nitrates, diuretics, histamine H2 receptor blockers, HMG-CoA reductase inhibitors, NSAIDs, quinolones and benzodiazepines, no clinically significant drug interactions were observed.
Drug interactions
No clinically significant interactions were found between finasteride and propranolol, digoxin, glyburide, warfarin, theophylline and antipyrine.
Apparently, finasteride does not have a significant effect on the cytochrome P450 enzyme system and, accordingly, does not affect the pharmacokinetic parameters of drugs metabolized by microsomal liver enzymes.
With simultaneous use of finasteride with ACE inhibitors, alpha-blockers, beta-blockers, calcium channel blockers, nitrates, diuretics, histamine H2 receptor blockers, HMG-CoA reductase inhibitors, NSAIDs, quinolones and benzodiazepines, no clinically significant drug interactions were observed.