Pharmacological properties of the drug Dutasteride
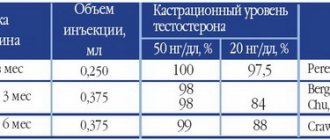
A drug for the treatment of benign prostatic hyperplasia. Suppresses the activity of 5α-reductase isoenzymes type 1 and 2, which are responsible for the conversion of testosterone to 5α-dihydrotestosterone (DHT). Dihydrotestosterone is the main androgen responsible for hyperplasia of the glandular tissue of the prostate gland. The maximum effect of dutasteride on reducing dihydrotestosterone concentrations is dose-dependent and is observed 1–2 weeks after the start of treatment. After 1 and 2 weeks of taking dutasteride at a dose of 0.5 mg/day, the average concentration of dihydrotestosterone in the blood serum decreases by 85 and 90%, respectively. Treatment with dutasteride reduces the size of the prostate gland, improves urination and reduces the risk of acute urinary retention and the need for surgical treatment. After a single dose of 500 mcg, the maximum concentration of dutasteride in the blood serum is achieved within 1–3 hours. With a 2-hour IV infusion, the absolute bioavailability is about 60%. The bioavailability of dutasteride is independent of food intake. Binding to blood plasma proteins is high - more than 99.5%. Distribution volume - 300–500 l. When taken daily, the serum concentration of dutasteride reaches 65% of the steady-state concentration after 1 month and approximately 90% of this level after 3 months. The equilibrium concentration of dutasteride in the blood serum, which is approximately 40 ng/ml, is achieved after 6 months of daily administration at a dose of 500 mcg. In sperm, as in blood serum, the equilibrium concentration of dutasteride is also achieved after 6 months. After 52 weeks of treatment, the concentration of dutasteride in sperm averages 3.4 ng/ml (0.4–14 ng/ml). Approximately 11.5% of dutasteride enters the sperm from the blood. In vitro, dutasteride is metabolized by the CYP 3A2 isoenzyme to form two minor monohydroxylated metabolites; however, it is not affected by the isoenzymes CYP 2C9, CYP 2C19 and CYP 2D6. After reaching the equilibrium concentration of dutasteride in the blood serum, the mass spectrometric method reveals unchanged dutasteride, 3 major metabolites (4′ hydroxydutasteride, 1,2 - dihydrodutasteride and 6 - hydroxydutasteride) and 2 minor metabolites. Dutasteride undergoes extensive metabolism. After taking the drug orally at a dose of 500 mcg/day until a steady state is reached, 1–15.4% (on average 5.4%) of the dose taken is excreted unchanged in the feces. The remainder of the dose is excreted as 4 major metabolites, accounting for 39, 21, 7, and 7%, respectively, and 6 remaining metabolites (each accounting for less than 5%). Trace amounts of unchanged dutasteride are excreted in the urine (less than 0.1% of the dose). When taking dutasteride in therapeutic doses, its terminal half-life is 3-5 weeks. Dutasteride is detectable in blood serum (at concentrations greater than 0.1 ng/ml) up to 4–6 months after stopping its use. The pharmacokinetics of dutasteride can be described as a first-order absorption process and two parallel elimination processes, one saturable (i.e., concentration-dependent) and one non-saturable (i.e., concentration-independent). At low serum concentrations (less than 3 ng/ml), dutasteride is rapidly eliminated by both elimination processes. After a single dose of 5 mg or less, dutasteride is quickly eliminated from the body and has a short half-life of 3–5 days. At serum concentrations greater than 3 ng/mL, the clearance of dutasteride is 0.35–0.58 L/h, with elimination occurring primarily through a linear, nonsaturable process with a terminal half-life of 3–5 weeks. At therapeutic concentrations with a daily dose of 500 mcg, slower clearance of dutasteride predominates; the total clearance is linear and independent of concentration. After a single dose of 5 mg, there were no significant differences in the pharmacokinetic parameters of dutasteride such as AUC, maximum concentration and half-life between different age groups. There were no significant differences in the degree of reduction in DHT levels between age groups. In this regard, no dose adjustment of dutasteride is required in elderly patients.
Use of the drug Dutasteride
For adult men, including elderly patients, the recommended oral dose is 500 mcg once a day, regardless of meals. The effect occurs quite quickly, but treatment should be continued for at least 6 months in order to objectively assess the effectiveness of therapy. In case of impaired renal function, a dose reduction is not required (since when taking dutasteride at a dose of 500 mcg/day, less than 0.1% of the dose taken is excreted in the urine). Dutasteride should be used with caution in patients with impaired liver function, as it undergoes extensive metabolism in the liver and its half-life is 3-5 weeks.
Avodart
Use during pregnancy and breastfeeding
Effect on fertility
The effect of dutasteride at a daily dose of 500 mcg on sperm characteristics was studied in healthy volunteers aged 18-52 years. By week 52 of treatment, the mean percentage reductions in total sperm count, semen volume, and sperm motility were 23%, 26%, and 18%, respectively, from baseline. The concentration of sperm and their morphological characteristics did not change.
At 24 weeks of follow-up, the mean percentage change in total sperm count in the dutasteride group remained 23% lower than baseline. The mean value for all sperm parameters at all time points remained within the normal range and did not meet the specified criteria for a clinically significant change (30%), at week 52 of treatment, two volunteers in the dutasteride group had a more than 90% reduction in total sperm count compared with baseline, with partial recovery at 24 weeks of observation.
Thus, the clinical significance of the effect of dutasteride on sperm parameters and on individual patient fertility is unknown.
Pregnancy
Dutasteride is contraindicated in women. Dutasteride has not been studied in women because Preclinical data suggest that suppression of DHT levels may inhibit the development of the external genitalia in the fetus.
Lactation
There is no data on the penetration of dutasteride into breast milk.
Use for liver dysfunction
The drug should be used with caution in patients with impaired liver function, because dutasteride undergoes intensive metabolism in the liver, and its T1/2 is 3-5 weeks.
The drug should be prescribed with caution in case of liver failure.
Use for renal impairment
In case of impaired renal function, a reduction in the dose of the drug is not required (since when taking the drug at a dose of 500 mcg/day, less than 0.1% of the dose is excreted in the urine).
Use in children
Avodart® is contraindicated in children.
Use in elderly patients
No dose adjustment is required.
special instructions
Dutasteride is absorbed through the skin, so women and children should avoid contact with damaged capsules. In case of contact with damaged capsules, immediately wash the affected area of skin with soap and water.
Liver dysfunction
There are currently no data on the use of Avodart® in patients with impaired liver function. Since dutasteride is extensively metabolized and its half-life is 3-5 weeks, caution must be exercised when treating patients with impaired liver function with Avodart®.
Heart failure with combined use of dutasteride and tamsulosin
In two 4-year clinical studies, the incidence of heart failure was higher in patients receiving the combination of dutasteride and an alpha1-blocker, primarily tamsulosin, than in patients not receiving the combination treatment. In these two studies, the incidence of heart failure remained low (≤ 1%) with some variability between them. But in general, there were no differences in the incidence of side effects from the cardiovascular system. A cause-and-effect relationship between treatment with dutasteride (as monotherapy or in combination with an alpha1-blocker) and the development of heart failure has not been established.
Impact on the detection of prostate-specific antigen (PSA) and prostate cancer (PCa)
Patients should undergo a digital rectal examination, as well as other methods of examining the prostate gland, before starting treatment with dutasteride and periodically repeat them during treatment to exclude the development of prostate cancer.
Determination of serum PSA concentration is an important component of screening for PCa. After 6 months of dutasteride therapy, the mean serum PSA level decreases by approximately 50%. Patients taking dutasteride should have a new baseline PSA level determined after 6 months of therapy. In the future, it is recommended to regularly monitor PSA levels.
The use of dutasteride does not affect the diagnostic value of PSA level as a marker of prostate cancer. Any confirmed increase in PSA levels relative to the nadir during dutasteride treatment may indicate the development of prostate cancer (particularly high-grade Gleason prostate cancer) or non-adherence to dutasteride therapy and should be carefully assessed, even if these PSA levels remain unchanged. within normal values for this age category of patients not taking 5α-reductase inhibitors.
Total PSA levels return to baseline within 6 months after discontinuation of dutasteride.
The ratio of free PSA to total remains constant even during dutasteride therapy. If the determination of the percentage of free PSA fraction is additionally used to detect prostate cancer in men receiving dutasteride, no correction of this value is required.
The effect of long-term use of dutasteride on the development of breast cancer in men
There was no effect of long-term use of dutasteride on the development of breast cancer in men.
PCa and high-grade tumors
The 4-year study (REDUCE) compared placebo and dutasteride in 8231 volunteers aged 50 to 75 years with a negative biopsy for prostate cancer and a PSA level of 2.5 ng/ml to 10 ng/ml at initial examination. .
During the study, 6,706 patients underwent a puncture biopsy of the prostate gland and, based on the results obtained, the degree of malignancy of prostate cancer was determined according to the Gleason score. 1517 patients were diagnosed with prostate cancer during the study. In the majority of cases, both in the dutasteride group and in the placebo group, well-differentiated prostate cancer was diagnosed (Gleason score 5-6). There was no difference in the number of cases of PCa with a Gleason score of 7-10 in the dutasteride group and the placebo group (p = 0.81).
After 4 years, there were more cases of PCa with a Gleason score of 8-10 in the dutasteride group (n = 29; 0.9%) compared with the placebo group (n = 19; 0.6%) (p = 0.15). When assessing biopsy data over 1-2 years, the number of patients diagnosed with PCa with a Gleason score of 8-10 was comparable in the dutasteride (n = 17; 0.5%) and placebo (n = 18; 0.5%) groups. When assessing biopsy data at 3-4 years, more cases of PCa with a Gleason score of 8-10 were diagnosed in the dutasteride group (n = 12; 0.5%) compared with the placebo group (n = 1; < 0.1%) (p = 0.0035). The percentage of patients diagnosed with PCa with a Gleason score of 8-10 was stable over all time periods (1-2 and 3-4 years) in the dutasteride group (0.5% in each period), while in In the placebo group, the percentage of patients diagnosed with PCa with a score of 8-10 was lower in years 3-4 than in years 1-2 (<0.1% compared with 0.5%, respectively).
In a 4-year study (CombAT) of patients with BPH, in which prostate biopsy was not mandated for all participants and all PCa diagnoses were based on indicated biopsy, PCa with a Gleason score of 8–10 was diagnosed in 8 patients (<0.5%) when taking dutasteride, 11 patients (<0.7%) when taking tamsulosin and 5 patients (<0.3%) when taking combination therapy with dutasteride and tamsulosin.
A cause-and-effect relationship between taking dutasteride and the development of high-grade prostate cancer has not been established.
Men taking dutasteride should undergo regular screening to assess their risk of developing prostate cancer, including PSA levels.
Impact on the ability to drive vehicles and operate machinery
Taking dutasteride does not affect driving or operating machinery.
Special instructions for the use of Dutasteride
Dutasteride is absorbed through the skin, so women and children should avoid contact with damaged capsules. In case of contact with damaged capsules, immediately wash the affected area of skin with soap and water. In patients with benign prostatic hyperplasia, it is necessary to conduct a digital rectal examination and other methods of examining the prostate gland before starting treatment with dutasteride and periodically repeat these studies during treatment to exclude the development of prostate cancer. Determination of prostate-specific antigen concentrations in blood serum is an important component of a set of studies aimed at detecting prostate cancer. Typically, additional examination is carried out in patients with a prostate-specific antigen concentration of more than 4 ng/ml; in such cases, a prostate biopsy may be indicated. A baseline prostate specific antigen level of less than 4 ng/ml in patients receiving dutasteride does not exclude the diagnosis of prostate cancer. After treatment with dutasteride for 6 months, there is a decrease in serum levels of prostate-specific antigen in patients with benign prostatic hyperplasia by approximately 50%, even in the presence of prostate cancer. Despite individual variability, a decrease in prostate-specific antigen levels of approximately 50% is observed across the entire range of initial prostate-specific antigen concentrations (from 1.5 to 10 ng/ml). Therefore, when interpreting the level of prostate-specific antigen in a man who has been receiving dutasteride for 6 months or more, the measured level should be multiplied by 2 and only then compared with normal levels in men not receiving dutasteride.
Dutasteride Bacter, 0.5 mg, capsules, 30 pcs.
Dutasteride is absorbed through the skin, so women and children should avoid contact with damaged capsules. In case of contact with damaged capsules, immediately wash the affected area of skin with soap and water. Fertility The effect of dutasteride at a daily dose of 0.5 mg on sperm characteristics was studied in healthy volunteers aged 18-52 years. At week 52 of treatment, the mean percentage reductions in total sperm count, semen volume, and sperm motility in the dutasteride group were 23%, 26%, and 18%, respectively, compared with baseline in the placebo group. . Sperm concentration and morphology did not change. At 24 weeks of follow-up, the mean percentage change in total sperm count in the dutasteride group remained 23% lower than baseline. The mean value for all sperm parameters at all time points remained within the normal range and did not meet the specified criteria for a clinically significant change (30%), at week 52 of treatment, two volunteers in the dutasteride group had a more than 90% reduction in total sperm count compared with baseline, with partial recovery at 24 weeks of observation. Thus, the clinical significance of the effect of dutasteride on sperm parameters and on individual patient fertility is unknown. Impaired liver function There are currently no data on the use of dutasteride in patients with impaired liver function. Since dutasteride is extensively metabolized and its half-life is 3-5 weeks, caution should be exercised when treating patients with impaired liver function with dutasteride. Heart failure with the combined use of dutasteride and tamsulosin In two 4-year clinical studies, the incidence of heart failure was higher in patients receiving the combination of dutasteride and an α-blocker, mainly tamsulosin, than in patients not receiving the combination treatment. In these two studies, the incidence of heart failure remained low (≤ 1%) with some variability between them. But in general, there were no differences in the incidence of side effects from the cardiovascular system. A causal relationship between treatment with dutasteride (as monotherapy or in combination with an α-blocker) and the development of heart failure has not been established. Effect on the detection of prostate-specific antigen (PSA) and prostate cancer (PCa) In patients, it is necessary to perform a digital rectal examination, as well as use other methods for examining the prostate gland, before starting treatment with dutasteride and repeat them periodically during treatment to exclude the development of prostate cancer. Determination of serum PSA concentration is an important component of screening for PCa. After 6 months of dutasteride therapy, the average serum PSA level decreases by approximately 50%. Patients taking dutasteride should have a new baseline PSA level determined after 6 months of therapy. In the future, it is recommended to regularly monitor PSA levels. When interpreting a PSA value in a patient taking dutasteride, the previous PSA value should be used for comparison. The use of dutasteride does not affect the diagnostic value of the PSA level as a marker of prostate cancer after determining a new baseline PSA level. Any confirmed increase in PSA levels relative to the nadir during dutasteride treatment may indicate the development of prostate cancer (particularly high-grade Gleason prostate cancer) or non-adherence to dutasteride therapy and should be carefully assessed, even if these PSA levels remain unchanged. within normal values for this age category of patients not taking 5α-reductase inhibitors. Total PSA levels return to baseline within 6 months after discontinuation of dutasteride. The ratio of free PSA to total remains constant even during dutasteride therapy. If the determination of the percentage of free PSA fraction is additionally used to detect prostate cancer in men receiving dutasteride, no correction of this value is required. PCa and high-grade tumors The 4-year study (REDUCE) compared placebo and dutasteride in 8231 volunteers aged 50 to 75 years with a negative biopsy for PCa and a PSA level of 2.5 ng/ml to 10.0 ng/ml at the initial examination. During the study, 6,706 patients underwent a puncture biopsy of the prostate gland, and based on the results obtained, the degree of malignancy of prostate cancer was determined according to the Gleason score. 1517 patients were diagnosed with prostate cancer during the study. In the majority of cases, both in the dutasteride group and in the placebo group, well-differentiated prostate cancer was diagnosed (Gleason score 5-6). There was no difference in Gleason score 7-10 PCa scores between the dutasteride group and the placebo group (p=0.81). After 4 years, there were more cases of PCa with a Gleason score of 8-10 in the dutasteride group (n = 29; 0.9%) compared with the placebo group (n = 19; 0.6%) (p = 0. 15). When assessing biopsy data for years 1-2, the number of patients diagnosed with PCa with a Gleason score of 8-10 was comparable in the dutasteride (n = 17; 0.5%) and placebo (n = 18; 0.5) groups. %). When assessing biopsy data for years 3-4, more cases of PCa with a Gleason score of 8-10 were diagnosed in the dutasteride group (n = 12; 0.5%) compared with the placebo group (n = 1; <0). .1%) (p = 0.0035). The percentage of patients diagnosed with PCa with a Gleason score of 8-10 was stable over all time periods (1-2 and 3-4 years) in the dutasteride group (0.5% in each period), while As in the placebo group, the percentage of patients diagnosed with PCa with a score of 8-10 points was lower over the course of 3-4 years than in years 1-2 (<0.1% compared with 0.5%, respectively). In a 4-year study (CombAT) of patients with BPH, in which prostate biopsy was not mandated for all participants and all prostate diagnoses were based on indicated biopsy, prostate cancer with a Gleason score of 8–10 was diagnosed in 8 patients (<0.5%) when taking dutasteride, 11 patients (<0.7%) when taking tamsulosin, and 5 patients (<0.3%) during combination therapy with dutasteride and tamsulosin. A cause-and-effect relationship between taking dutasteride and the development of high-grade prostate cancer has not been established. Men taking dutasteride should undergo regular screening to assess their risk of developing prostate cancer, including PSA levels. Breast cancer in men Breast cancer has been reported in clinical studies and during post-marketing surveillance in men taking dutasteride. Patients should be warned that if any changes occur in the breast tissue, such as the appearance of nodules or nipple discharge, they should immediately report this to their doctor. In clinical studies examining the effect of dutasteride monotherapy for BPH (3374 patient-years), 2 cases of breast cancer were identified during dutasteride therapy (at 10 weeks and 11 months) and 1 case in a patient receiving placebo. In a follow-up clinical trial that included 8,231 men aged 50 to 75 years with a negative biopsy for prostate cancer and a PSA level between 2.5 ng/mL and 10.0 ng/mL (17,489 patient-years), who received dutasteride and patients (5027 patient-years) who received combination therapy with dutasteride and tamsulosin, there were no cases of breast cancer in any of the comparison groups. It is currently unclear whether there is a cause-and-effect relationship between the occurrence of breast cancer in men and long-term use of dutasteride.
Drug interactions Dutasteride
Since dutasteride is metabolized by the CYP3A4 isoenzyme, dutasteride blood concentrations may increase in the presence of CYP3A4 inhibitors. When dutasteride is used concomitantly with the CYP3A4 inhibitors verapamil and diltiazem, a decrease in its clearance is observed. However, amlodipine, another calcium channel blocker, does not reduce the clearance of dutasteride. With the simultaneous use of dutasteride and CYP3A4 inhibitors, the decrease in dutasteride clearance and the subsequent increase in its concentration in the blood is not significant due to its wide therapeutic range, so there is no need to reduce its dose. In vitro, CYP 1A2, CYP 2C9, CYP 2C19 and CYP 2D6 isoenzymes are not involved in the metabolism of dutasteride in humans; dutasteride does not inhibit enzymes of the human cytochrome P450 system involved in the metabolism of drugs. When dutasteride is used simultaneously with lipid-lowering drugs, ACE inhibitors, β-adrenergic receptor blockers, calcium channel blockers, corticosteroids, diuretics, NSAIDs, PDE-5 inhibitors and quinolone derivatives, no significant drug interactions are observed. When dutasteride was used simultaneously with tamsulosin or terazosin for 2 weeks, no pharmacokinetic or pharmacodynamic interaction was detected. With simultaneous use of dutasteride and tamsulosin for 9 months, this combination was shown to be well tolerated. Dutasteride does not displace warfarin, diazepam and phenytoin from the sites of their binding to plasma proteins, and these drugs, in turn, do not displace dutasteride. Warfarin, digoxin and cholestyramine do not interact with dutasteride.