Magnefar B6 Biopharm tablets 500mg/5mg No. 10x6
Name
Magnefar B6 biopharm.
Release forms
Pills.
INN
Magnesium hydroaspartate tetrahydrate + pyridoxine hydrochloride.
FTG
Magnesium preparation.
Description
Oblong biconvex tablets, white, with a smooth surface, scored on one side. The score is designed to make it easier to break the tablet if you have difficulty swallowing it whole.
Compound
Each tablet contains: active ingredients: magnesium hydroaspartate tetrahydrate 500 mg, which corresponds to 34 mg of magnesium ions, and pyridoxine hydrochloride (vitamin B6) 5 mg. Excipients: microcrystalline cellulose, magnesium stearate, hypromelose.
Pharmacotherapeutic group
Mineral supplements. Other mineral supplements. Magnesium-based products. ATS code: A12CC
Indications for use
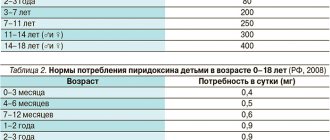
Treatment of confirmed magnesium deficiency in the body - both isolated and associated with other deficiency conditions. A combination of a certain number of the following symptoms may indicate magnesium deficiency: – nervousness, irritability, mild anxiety, transient fatigue, minor sleep disturbances; – signs of anxiety, such as gastrointestinal cramps or rapid heartbeat (if the heart is healthy); – muscle cramps, tingling sensation. Taking magnesium may help relieve these symptoms. If no improvement is observed after one month of treatment, continuation of monotherapy with this drug is not advisable.
Contraindications
– if the patient has a hypersensitivity to magnesium hydroaspartate, pyridoxine hydrochloride (vitamin B6) or any other component of this medicine, if the patient has diarrhea, – if the patient has hypermagnesemia (increased concentration of magnesium in the blood), – if the patient hypervitaminosis B6 is detected (increased concentration of vitamin B6 in the blood), - if the patient has severe renal failure, - if the patient has severe arterial hypotension, - if the patient has atrioventricular block, - if the patient has a disease called myasthenia gravis (acquired a chronic disease characterized by rapid fatigue and weakening of skeletal muscles) – children under 6 years of age. Cautions for use - the medicine should not be taken on an empty stomach, - do not exceed the recommended dose, as this can lead to diarrhea, which can cause dehydration of the body that is unsafe in its consequences, - with long-term use of the medicine, it is necessary to monitor the concentration of magnesium in the plasma. In cases of concomitant calcium deficiency, magnesium deficiency must be corrected before supplemental calcium is administered. Take with caution with digitalis glycosides (prescribed for heart failure). This dosage form is not prescribed to children under 6 years of age.
Drug interactions and other interactions
Contraindicated combinations With levodopa: the activity of levodopa is inhibited by pyridoxine (unless taking this drug is combined with taking inhibitors of peripheral aromatic L-amino acid decarboxylase). Any amount of pyridoxine should be avoided unless levodopa is taken in combination with peripheral aromatic L-amino acid decarboxylase inhibitors. Not recommended combinations The simultaneous use of drugs containing phosphates or calcium salts may impair the absorption of magnesium in the intestine. Combinations that need to be taken into account When prescribing fluoroquinolones and tetracyclines orally, it is necessary to maintain an interval of at least three hours between ingestion of them and Magnefar B6 Biopharm, since magnesium preparations reduce the absorption of tetracyclines and fluoroquinolones. The use of cycloserine, hydralazine, isoniazid, penicillamine leads to a decrease in the content of vitamin B6 in the body. Diuretics (furosemide, ethacrynic acid) increase the excretion of magnesium in the urine. Long-term use of potassium-sparing diuretics can increase the reabsorption of magnesium in the renal tubules and lead to hypermagnesemia. Other medications containing magnesium, such as laxatives and stomach acid suppressants, taken concomitantly with magnesium hydroaspartate may cause symptoms of magnesium toxicity, especially in individuals with kidney failure.
Pregnancy and lactation period
Clinical experience with the use of the drug in a sufficient number of pregnant women has not revealed any adverse effects on the occurrence of fetal defects or fetotoxic effects. The drug can be used during pregnancy only if necessary, on the recommendation of a doctor. Magnesium passes into breast milk. The use of the drug should be avoided during breastfeeding.
Impact on the ability to drive vehicles and operate machinery
Does not affect.
Directions for use and doses
Magnefar B6 Biopharm tablets are taken orally after meals with a small amount of liquid. The dividing line on the tablet is designed to break the tablet to make it easier to swallow. Adults are recommended to take 6-8 tablets per day. Children over 6 years old (with body weight more than 20 kg) – 4-6 tablets per day. The daily dose should be divided into 2-3 doses. There are no data on the maximum permissible daily dose. Renal failure: The drug is contraindicated in patients with severe renal failure. Liver failure: No dose change required. Duration of treatment The duration of treatment is usually one month. Treatment should be stopped immediately after normalization of magnesium levels in the blood. If you miss the next dose of Magnefar B6 Biopharm, treatment should be continued, following the indicated dosage method for Magnefar B6 Biopharm. Do not take a double dose to make up for a missed dose.
Overdose
Overdose symptoms rarely occur in patients with normal renal function. Taking medication in doses significantly higher than recommended can cause: allergic reactions, nausea, vomiting, abdominal pain, diarrhea and dehydration, depression of the central nervous system, decreased reflexes, ECG abnormalities. If diarrhea occurs, it is necessary to reduce the daily dose or temporarily stop taking the drug. Other symptoms of overdose: asystole (lack of heartbeat), bradycardia (rare heartbeat), drowsiness, coma, hypotension (low blood pressure), muscle paralysis, renal failure, respiratory failure. Treatment of overdose: it is necessary to exclude drugs containing magnesium, maintain breathing, rehydration, forced diuresis. In case of renal failure - hemodialysis or peritoneal dialysis. A specific antidote is calcium gluconate. If you take a dose higher than recommended, you should immediately consult a doctor.
Side effect
Like all medicines, Magnefar B6 Biopharm can cause side effects, although not everyone gets them. The following undesirable reactions have been recorded: Rare - may occur in less than 1 person in 1,000: abdominal pain, nausea and diarrhea, which usually go away on their own, possible atrioventricular conduction disturbance, redness of the skin Very rare - may occur in less than 1 in 10,000 people: allergic reactions, including skin reactions Reporting adverse reactions If you experience any adverse reactions, consult your doctor. This recommendation applies to any possible adverse reactions, including those not listed in the package insert. You can also report adverse reactions to the Adverse Drug Events Information Database, including reports of drug failure. By reporting adverse reactions, you can help provide more information about the safety of the drug.
Storage conditions
Store at temperatures from 15°C to 25°C, protected from light and moisture. Keep out of the reach of children.
Shelf life
3 years. Do not use the drug after its expiration date.
Vacation conditions
Over the counter.
Package
10 tablets in blisters made of PVC/Al foil. 6 blisters with an insert are placed in cardboard packs.
Buy Magnefar B6 Biopharm tab. 500mg/5mg per bl. in pack No. 10x6 in the pharmacy
Price for Magnefar B6 Biopharm tab. 500mg/5mg per bl. in pack №10x6
Instructions for use for Magnefar B6 Biopharm tab. 500mg/5mg per bl. in pack №10x6
Magnefar
Name: Magnefar Pharmacological action: Magnefar is a complex preparation containing pyridoxine hydrochloride and magnesium. Magnefar helps restore magnesium levels in patients with magnesium deficiency, as well as normalize magnesium-dependent processes. Magnesium takes part in a number of metabolic processes in the body, including ATP-dependent enzymatic reactions, hormone metabolism, and stabilization of cell membranes. Magnesium is necessary for the normal functioning of the nervous, respiratory, cardiovascular, musculoskeletal and hematopoietic systems. Magnesium plays an important role in the functioning of the visual and auditory nerves. Magnesium maintains the normal state of the myocardium, including under conditions of hypoxia, has anticoagulant properties, and prevents hardening of blood vessels. In addition, magnesium has some calming activity and regulates neuromuscular excitability.
Pyridoxine hydrochloride is a B vitamin that regulates lipid, protein and carbohydrate metabolism. Pyridoxine hydrochloride takes part in the formation of hemoglobin. Magnesium and pyridoxine hydrochloride mutually enhance each other's effects, in addition, pyridoxine hydrochloride improves the intestinal absorption of magnesium and its penetration into cells. Pyridoxine is metabolized in the liver with the participation of magnesium to form the active substance - pyridoxal-5-phosphate.
Indications for use: Magnefar is intended for the treatment of patients with primary and secondary magnesium deficiency, including congenital magnesium deficiency, insufficient intake of magnesium from food, taking medications that can lead to magnesium deficiency, as well as a number of diseases in which the need for magnesium is increased or its excretion from the body is increased. Magnefar is also prescribed to patients suffering from excessive excitability, increased irritability, sleep disturbances, muscle spasms and fatigue (physical and intellectual), which are caused by magnesium deficiency.
Directions for use: Magnefar is intended for oral use. It is recommended to take the tablets with meals, without chewing and with plenty of drinking water. The duration of therapy and dose of Magnefar is determined by the doctor, taking into account the level of magnesium deficiency. Adults and adolescents are usually recommended to take 1-2 tablets of Magnefar three times a day. For children from 6 to 12 years old, as a rule, it is recommended to take 1 tablet of Magnefar twice or three times a day.
As a preventative measure, the drug Magnefar is prescribed in a daily dose of 1-2 tablets. Therapy should be continued until plasma magnesium concentrations normalize. If necessary, the course of therapy is repeated 1-2 times a year. If the patient has a concomitant calcium deficiency, then the level of magnesium in the body should be adjusted before starting calcium therapy.
Side effects: Magnefar, as a rule, does not cause the development of undesirable effects. However, the possibility of side effects cannot be ruled out (especially when taking high doses of magnesium). In particular, patients may develop the following side effects due to taking the drug Magnefar: From the cardiovascular system: arrhythmia, atrioventricular block, decreased blood pressure.
From the gastrointestinal tract: flatulence, pain and discomfort in the epigastric region, vomiting, nausea. Allergic reactions: urticaria, skin hyperemia. Side effects disappear after discontinuation of the drug Magnefar and do not require additional therapy.
Contraindications: Magnefar is not prescribed to patients with individual intolerance to the components of the drug. Magnefar is not used for the treatment of patients with hypervitaminosis B6 and elevated levels of magnesium in the blood plasma, as well as patients suffering from severe renal impairment, atrioventricular block, arterial hypotension (with systolic pressure less than 90 mmHg), myasthenia gravis and disorders intestinal absorption (including chronic diarrhea).
Magnefar should not be prescribed to patients with Parkinson's disease who are receiving levodopa without combination with peripheral levodopa decarboxylase inhibitors. Magnefar is not used in pediatric practice to treat children under 6 years of age. Magnefar is prescribed with caution to patients suffering from moderate renal impairment.
Pregnancy: Magnefar can be prescribed during pregnancy if the benefits to the mother outweigh the possible risks to the fetus. Magnefar should not be used in combination with vitamin-mineral complex preparations for pregnant women. During lactation, taking Magnefar is possible only after the issue of possible interruption of breastfeeding has been resolved.
Interaction with other drugs: There is a mutual decrease in intestinal absorption with the combined use of the drug Magnefar with theophylline, tetracyclines, fluoroquinolone antibiotics, anticoagulant agents warfarin derivatives, iron preparations, phosphates, as well as calcium salts (if it is necessary to use these drugs in combination, the interval between their receptions for at least 3 hours). There is an increased need for pyridoxine hydrochloride in patients receiving therapy with hydralazine, cycloserine, isoniazid, oral contraceptives and penicillamine. Pyridoxine hydrochloride, when used in combination, can induce the metabolism of levodopa and reduce the severity of its therapeutic effect (the maximum recommended daily dose of Magnefar for patients receiving levodopa is 1 tablet).
Diuretics may decrease the half-life of magnesium by increasing its excretion by the kidneys. However, with prolonged use of potassium-sparing diuretics simultaneously with the drug Magnefar, patients may develop hypermagnesemia due to increased resorption of magnesium in the renal tubules. Caution should be exercised when using Magnefar simultaneously with other magnesium-containing drugs (including laxatives and antacids). Caution should be exercised when prescribing Magnefar to patients receiving digitalis preparations.
Overdose: The use of high doses of Magnefar, as a rule, does not cause the development of toxic effects. Mainly when using excessive doses of magnesium in patients with impaired renal function or when using the drug Magnefar in doses that are tens of times higher than recommended, the development of vomiting, diarrhea, arterial hypotension, arrhythmia, myasthenia gravis, respiratory depression, and pain in the extremities is possible. In addition, with magnesium intoxication, it is possible to develop disturbances in temperature sensitivity and decreased tendon reflexes.
Calcium salts are a specific antidote for magnesium. In case of an overdose of Magnefar, gastric lavage is performed and enterosorbents are prescribed. In case of severe intoxication, intravenous administration of calcium salts (for example, calcium gluconate) at a dose of 2.5-5 mmol per 1 kg of body weight (in terms of pure calcium) is indicated.
Release form: Magnefar tablets in blister packs of 10 pieces, 6 blister packs are included in a cardboard pack.
Storage conditions: The drug Magnefar should be stored for no more than 3 years after release in rooms with a temperature that does not exceed 25 degrees Celsius.
Synonyms: Magvit, Magnicum, Magne-B6.
Composition: 1 tablet of Magnefar contains: Magnesium hydroaspartate tetrohydrate – 500 mg (in terms of magnesium – 34 mg); Pyridoxine hydrochloride – 5 mg; Additional ingredients.
Attention! The description of the drug " Magnefar " on this page is a simplified and expanded version of the official instructions for use. Before purchasing or using the drug, you should consult your doctor and read the instructions approved by the manufacturer. Information about the drug is provided for informational purposes only and should not be used as a guide to self-medication. Only a doctor can decide to prescribe the drug, as well as determine the dose and methods of its use.
Magnefar - instructions for use, analogues, composition, indications
About the drug:
Magnefar is a complex preparation containing pyridoxine hydrochloride and magnesium. Magnefar helps restore magnesium levels in patients with magnesium deficiency, as well as normalize magnesium-dependent processes.
Indications and dosage:
Magnefar is intended for the treatment of patients with primary and secondary magnesium deficiency, including congenital magnesium deficiency, insufficient intake of magnesium from food, taking medications that can lead to magnesium deficiency, as well as a number of diseases in which the need for magnesium is increased or its excretion is increased from the body.
Magnefar is also prescribed to patients suffering from excessive excitability, increased irritability, sleep disturbances, muscle spasms and fatigue (physical and intellectual), which are caused by magnesium deficiency.
Magnefar is intended for oral use.
It is recommended to take the tablets with meals, without chewing and with plenty of drinking water.
The duration of therapy and dose of Magnefar is determined by the doctor, taking into account the level of magnesium deficiency.
Adults and adolescents are usually recommended to take 1-2 tablets of Magnefar three times a day.
For children from 6 to 12 years old, as a rule, it is recommended to take 1 tablet of Magnefar twice or three times a day.
As a preventative measure, the drug Magnefar is prescribed in a daily dose of 1-2 tablets.
Therapy should be continued until plasma magnesium concentrations normalize.
If necessary, the course of therapy is repeated 1-2 times a year.
If the patient has a concomitant calcium deficiency, then the level of magnesium in the body should be adjusted before starting calcium therapy.
Overdose:
The use of high doses of Magnefar, as a rule, does not cause the development of toxic effects.
Mainly when using excessive doses of magnesium in patients with impaired renal function or when using the drug Magnefar in doses that are tens of times higher than recommended, the development of vomiting, diarrhea, arterial hypotension, arrhythmia, myasthenia gravis, respiratory depression, and pain in the extremities is possible.
In addition, with magnesium intoxication, it is possible to develop disturbances in temperature sensitivity and decreased tendon reflexes.
Calcium salts are a specific antidote for magnesium.
In case of an overdose of Magnefar, gastric lavage is performed and enterosorbents are prescribed.
In case of severe intoxication, intravenous administration of calcium salts (for example, calcium gluconate) at a dose of 2.5-5 mmol per 1 kg of body weight (in terms of pure calcium) is indicated.
Side effects:
Magnefar, as a rule, does not cause the development of undesirable effects. However, the possibility of side effects cannot be ruled out (especially when taking high doses of magnesium). In particular, patients may develop the following side effects caused by taking the drug Magnefar:
- From the cardiovascular system: arrhythmia, atrioventricular block, decreased blood pressure.
- From the gastrointestinal tract: flatulence, pain and discomfort in the epigastric region, vomiting, nausea.
- Allergic reactions: urticaria, skin hyperemia.
Side effects disappear after discontinuation of the drug Magnefar and do not require additional therapy.
Contraindications:
Magnefar is not prescribed to patients with individual intolerance to the components of the drug.
Magnefar is not used for the treatment of patients with hypervitaminosis B6 and elevated levels of magnesium in the blood plasma, as well as patients suffering from severe renal impairment, atrioventricular block, arterial hypotension (with systolic pressure less than 90 mmHg), myasthenia gravis and disorders intestinal absorption (including chronic diarrhea).
Magnefar should not be prescribed to patients with Parkinson's disease who are receiving levodopa without combination with peripheral levodopa decarboxylase inhibitors.
Magnefar is not used in pediatric practice to treat children under 6 years of age.
Magnefar is prescribed with caution to patients suffering from moderate renal impairment.
Magnefar can be prescribed during pregnancy if the benefits to the mother outweigh the possible risks to the fetus.
Magnefar should not be used in combination with vitamin-mineral complex preparations for pregnant women.
During lactation, taking Magnefar is possible only after the issue of possible interruption of breastfeeding has been resolved.
Interaction with other drugs and alcohol:
There is a mutual decrease in intestinal absorption with the combined use of the drug Magnefar with theophylline, tetracyclines, fluoroquinolone antibiotics, anticoagulant agents warfarin derivatives, iron preparations, phosphates, as well as calcium salts (if combined use of these drugs is necessary, an interval between doses of at least 3 hours should be observed ).
There is an increased need for pyridoxine hydrochloride in patients receiving therapy with hydralazine, cycloserine, isoniazid, oral contraceptives and penicillamine.
Pyridoxine hydrochloride, when used in combination, can induce the metabolism of levodopa and reduce the severity of its therapeutic effect (the maximum recommended daily dose of Magnefar for patients receiving levodopa is 1 tablet).
Diuretics may decrease the half-life of magnesium by increasing its excretion by the kidneys.
However, with prolonged use of potassium-sparing diuretics simultaneously with the drug Magnefar, patients may develop hypermagnesemia due to increased resorption of magnesium in the renal tubules.
Caution should be exercised when using Magnefar simultaneously with other magnesium-containing drugs (including laxatives and antacids).
Caution should be exercised when prescribing Magnefar to patients receiving digitalis preparations.
Composition and properties:
1 tablet of Magnefar contains:
- Magnesium hydroaspartate tetrohydrate – 500 mg (in terms of magnesium – 34 mg)
- Pyridoxine hydrochloride – 5 mg
- Additional Ingredients
Pharmachologic effect:
Magnefar is a complex preparation containing pyridoxine hydrochloride and magnesium. Magnefar helps restore magnesium levels in patients with magnesium deficiency, as well as normalize magnesium-dependent processes.
Magnesium takes part in a number of metabolic processes in the body, including ATP-dependent enzymatic reactions, hormone metabolism, and stabilization of cell membranes.
Magnesium is necessary for the normal functioning of the nervous, respiratory, cardiovascular, musculoskeletal and hematopoietic systems.
Magnesium plays an important role in the functioning of the visual and auditory nerves. Magnesium maintains the normal state of the myocardium, including under conditions of hypoxia, has anticoagulant properties, and prevents hardening of blood vessels.
In addition, magnesium has some calming activity and regulates neuromuscular excitability.
Pyridoxine hydrochloride is a B vitamin that regulates lipid, protein and carbohydrate metabolism.
Pyridoxine hydrochloride takes part in the formation of hemoglobin.
Magnesium and pyridoxine hydrochloride mutually enhance each other's effects, in addition, pyridoxine hydrochloride improves the intestinal absorption of magnesium and its penetration into cells.
Pyridoxine is metabolized in the liver with the participation of magnesium to form the active substance - pyridoxal-5-phosphate.
Release form:
Magnefar tablets in blister packs of 10 pieces, 6 blister packs are included in a cardboard pack.
Storage conditions:
The drug Magnefar should be stored for no more than 3 years after release in rooms with a temperature that does not exceed 25 degrees Celsius.
general information
- Sales form:
over-the-counter
- Manufacturer:
Piluli
- Pharm. group:
Other mineral supplements. Magnesium preparations.