Magnesium oxide is a substance used in sports, medicine and the food industry. Athletes and climbers use it on their hands to prevent slipping. Doctors and cosmetologists value magnesium oxide for its anti-inflammatory and antibactericidal effects. It is also present in some of our products as a food additive E530. Isn't this dangerous?
Chemical properties
The substance has a number of traditional names: burnt magnesia, periclase. Chemical formula of magnesium oxide: MgO. According to the pharmacopoeia, the compound appears as small white crystals that are insoluble in water. In pharmacology, the product is used in the form of a light, white, loose powder that has the ability to absorb water. The oxide boils at 3600 degrees Celsius, molecular weight = 40.3 grams per mole.
Chemical properties of Magnesium Oxide. The substance reacts with dilute substances, thereby forming salts. Magnesium oxide reacts with hot water to form hydroxide, but does not react with cold liquid. Magnesii oxydum (Magnesium Oxide in Latin) is obtained by roasting magnesite and dolomite . MgCO3 decomposes to oxide and carbon monoxide.
Application of the substance:
- used in industry in the production of refractory materials, cement, for purification from impurities of petroleum products, as a filler in the production of rubber products;
- as an abrasive for cleaning various surfaces in industry;
- in the food industry as an additive E530 ;
- athletes use burnt magnesia as a powder to prevent them from slipping off the equipment;
- in medicine - to neutralize hydrochloric or other acids in the stomach.
Applications of MgO in industry
Due to its high melting point, magnesium oxide is used in construction. In this case, the so-called “caustic magnesite”, which is obtained by firing natural magnesite, is highly valued. It is especially widely used in the creation of building materials such as xylitol, cement, and concrete. The chemical increases their fire resistance, so such materials are often used in the construction of industrial premises, residential and public buildings.
MgO is also used to create binders. However, its ability to absorb moisture allows the use of such materials and mixtures only for the construction of premises with predominantly dry operating conditions. In the automotive industry, this chemical compound is introduced into rubber compounds, and also for vulcanization as an activator of other accelerators.
Light magnesium oxide has abrasive properties, so it is often used in the electronics industry to clean sensitive surfaces. In addition, burnt magnesia has found its use in the following cases:
- is part of the protective layer in liquid crystal screens;
- used in paper production;
- included in heating elements in heating systems;
- Some types of petroleum products are purified using this chemical compound.
Another important property of the MgO compound is that it can be used to control the solubility of radionuclides. This quality is very useful in factories that process waste, the use of burnt magnesia in this case to maintain environmental balance.
Agriculture is considered a promising segment of magnesium oxide consumption today. Here it is used to prevent caking of fertilizers and is used as an independent additive to enrich the soil with magnesium. The lack of magnesium on farmland affects the products produced, so the use of such fertilizer is very important for the harvest. True, magnesium sulfate is most often used, since the latter is slightly cheaper than burnt magnesia.
This substance has also found its use in animal husbandry. Magnesium deficiency affects the health of livestock and the products derived from them. Farm animal diets typically contain sufficient amounts of all essential minerals and vitamins. But on pastures fertilized with potassium and nitrogen, there is sometimes a lack of Mg. Burnt magnesia is used to prevent and eliminate magnesium deficiency by introducing fertilizing.
Pharmacodynamics and pharmacokinetics
Magnesium Oxide, after entering the digestive tract under the influence of water, turns into hydroxide . The substance neutralizes hydrochloric acid and reduces the activity of digestive enzymes in general. After taking the medicine on an empty stomach, the antacid effect lasts for half an hour. When taken after meals – up to 4 hours.
Magnesium chloride is also formed in the stomach , which, upon penetration into the intestines, increases osmotic pressure, has a laxative effect, increasing intestinal motility.
The substance is not absorbed through the walls of the stomach and does not penetrate the systemic circulation. Secondary hypersecretion is not observed during treatment with the drug. The medicine does not cause alkalosis .
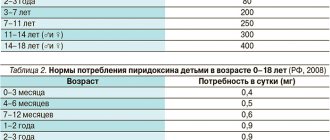
When combining the product with pyridoxine calcium oxalate formation decreases . This combination of lek. drugs prevents the formation of oxalate stones.
Magnesium oxide as a food additive
In food products, this component is usually found under the index E530. As a food additive, MgO is legal in the EU countries, Ukraine and Russia (data are not provided for other countries). Based on the degree of effect on the body, this substance is considered safe. In the food industry, magnesium oxide is valued mainly as an emulsifier and stabilizer.
Most often, E530 is found in the following products:
- milk powder (quantity 10 g/kg);
- dry cream (1000 mg/kg);
- chocolate and cocoa products (70 g/kg);
- edible oils;
- margarine, spread, butter.
The E530 component is added to food products to prevent clumping and caking. In the production of cooking fats and oils, this additive accelerates hydrogenation. Its presence in products does not indicate their poor quality, since the component is recognized as safe.
Indications for use
The medicine is prescribed:
- for acute gastritis , with exacerbation of chronic gastritis with increased or normal secretion of gastric acids;
- patients with exacerbation of gastric and duodenal ulcers ;
- patients with gastralgia , dyspepsia after taking medications, diet disorders, drinking alcohol, coffee or nicotine;
- with reflux esophagitis ;
- patients with pancreatitis ;
- for the treatment of constipation ;
- after poisoning with acids;
- in combination with other medications for the prevention of oxalate nephrourolithiasis .
Effect of E530 on the body: benefits and harms
Pharmaceuticals remain the main consumer of magnesium oxide. This chemical compound is added to medications and used for independent or complex treatment. The following pharmacological properties of E530 are valued in medicine: antacid, antiulcer, anti-inflammatory. Magnesium oxide also improves intestinal muscles.
Once in the digestive tract, burnt magnesia reacts with water and forms hydroxide. This substance reduces the effect of digestive enzymes, in particular neutralizes hydrochloric acid. Thanks to this, the E530 supplement is used for high acidity, against heartburn, for the treatment and prevention of ulcers. Also in the stomach, this substance forms magnesium chloride, a compound that improves intestinal motility and provides a laxative effect.
Preparations containing magnesium oxide are used to prevent the appearance of oxide stones. To do this, the substance is combined with pyridoxine. The additive does not cause an increase in the alkalinity of the blood and body tissues, and is also not able to penetrate the walls of the gastrointestinal tract into the bloodstream. Any effect on the body is possible only with the dosage prescribed by the doctor. That is, the amount of E530 that is in food does not affect the body.
Magnesium oxide helps with the following diseases:
- acute and chronic gastritis;
- exacerbation of duodenal and gastric ulcers;
- dyspepsia;
- pancreatitis;
- in case of poisoning with acids to neutralize their effect;
- to relieve constipation.
This supplement is also effective in preventing magnesium deficiency or replenishing reserves of this mineral. This component ensures the normal functioning of the nervous system, strengthens the bone structure and heart muscle. For these purposes, vitamin complexes are prescribed that contain MgO: Vitrum, Complivit Active, Multimax, Multi-tabs, Oligovit, etc.
Taking medications and vitamins with magnesium oxide for self-medication is unsafe, since in addition to the beneficial qualities, there are side effects and contraindications.
First of all, drugs with MgO are not prescribed to people with individual sensitivity to this substance. It is also dangerous to use them in case of hypermagnesemia - increased concentration of magnesium in the blood serum. An excess of Mg in the body inhibits the activity of the nervous and cardiovascular systems.
Best materials of the month
- Coronaviruses: SARS-CoV-2 (COVID-19)
- Antibiotics for the prevention and treatment of COVID-19: how effective are they?
- The most common "office" diseases
- Does vodka kill coronavirus?
- How to stay alive on our roads?
Such drugs should be used especially carefully by people with kidney disease, since disruption of their activity leads to hypermagnesemia. As prescribed by the doctor, medications containing magnesium oxide are often combined with aluminum antacids, which reduce the risk of side effects from the gastrointestinal tract and prolong the beneficial effects of the drugs.
There are no cases of overdose of burnt magnesia, as well as reviews from consumers. It is used for adults, some dosage forms are used in pediatrics, during breastfeeding and pregnancy, which indicates its relative safety and benefits for the body.
E530 can cause harm only in case of improper self-medication, excessive use of the substance, or combination with azithromycin (this combination is not recommended). Overdose of the drug occurs extremely rarely, only in cases of impaired digestion or functioning of other systems. In a healthy body, the required amount of magnesium is absorbed from the resulting burnt magnesium, and the rest is utilized without consequences for health.
Interaction
Magnesium Oxide reduces adverse reactions from taking antacids with aluminum and increases their duration of action.
When the drug is combined with indomethacin , the plasma concentration of the latter decreases and the irritating effect of the drug on the digestive tract decreases.
The medicine reduces the rate of absorption of nitrofurantoin , iron salts and tetracycline .
The substance slows down the absorption of azithromycin , reduces its maximum concentration in the blood and the time to reach this concentration. This combination is not recommended.
Cosmetic products with magnesium oxide
In the cosmetics industry, magnesium oxide is used as a stabilizer, buffer substance, and absorbent. But the main property that cosmetologists and dermatologists value is its texture. Its looseness, lightness and friability make it possible to successfully use burnt magnesia in the production of powders, talcs, blushes, etc. The presence of this component in the formulation of a cosmetic product allows you to avoid the formation of lumps even after a long period of use.
Magnesium oxide is found in the following products:
- decorative cosmetics;
- body sunscreens;
- hair and face masks;
- lotions against blackheads;
- children's talcs;
- deodorants and antiperspirants;
- shampoos.
If the product contains E530 or Magnesium Oxide, this does not in any way detract from the quality of the product itself. When used externally, this supplement is considered absolutely safe and has an anti-inflammatory and absorbent effect on the skin. Moreover, in combination with other chemical elements, its capabilities increase significantly. Most often, burnt magnesia is combined with zinc, which solves a very common problem - narrowing pores. Also, beneficial properties of magnesium oxide include skin whitening and sebum regulation (drying oily skin).
Physical properties:
| 200 | Physical properties | |
| 201 | Density* | 3.58 g/cm3 (at 20/25 °C and normal conditions, state of matter - solid) |
| 202 | Melting temperature* | 2852 °C (3125 K, 5166 °F) |
| 203 | Boiling temperature | 3600 °C (3870 K, 6510 °F) |
| 204 | Sublimation temperature | |
| 205 | Decomposition temperature | |
| 206 | Self-ignition temperature of a gas-air mixture | |
| 207 | Specific heat of fusion (enthalpy of fusion ΔHpl) | |
| 208 | Specific heat of evaporation (enthalpy of boiling ΔHboiling) | |
| 209 | Specific heat capacity at constant pressure | |
| 210 | Molar heat capacity | |
| 211 | Molar volume | |
| 212 | Thermal conductivity | 45-60 W/(mK) (at 25 °C) |
| 213 | Thermal expansion coefficient | |
| 214 | Thermal diffusivity coefficient | |
| 215 | Critical temperature | |
| 216 | Critical pressure | |
| 217 | Critical Density | |
| 218 | Triple point | |
| 219 | Solubility in water and other liquids | Water: 0.00062 (at 0 °C), water: 0.0086 (at 30 °C), ethanol: insoluble, acids: highly soluble |
| 220 | Vapor pressure (mmHg) | |
| 221 | Vapor pressure (Pa) | |
| 222 | Standard enthalpy of formation ΔH | -601.8 kJ/mol (at 298 K, for the state of matter - solid) |
| 223 | Standard Gibbs energy of formation ΔG | -569.6 kJ/mol (at 298 K, for the state of matter - solid) |
| 224 | Standard entropy of matter S | 26.9 J/(mol K) (at 298 K, for the state of matter - solid) |
| 225 | Standard molar heat capacity Cp | 37.8 J/(mol K) (at 298 K, for the state of matter - solid) |
| 226 | Enthalpy of dissociation ΔHdiss | |
| 227 | The dielectric constant | Diamagnetic material |
| 228 | Magnetic type | |
| 229 | Curie point | |
| 230 | Neel temperature | |
| 231 | Volume magnetic susceptibility | |
| 232 | Specific magnetic susceptibility | |
| 233 | Molar magnetic susceptibility | -10.2 10-6 cm3/mol (at 298 K) |
| 234 | Electric type | Dielectric |
| 235 | Electrical conductivity in the solid phase | |
| 236 | Electrical resistivity | |
| 237 | Superconductivity at temperature | |
| 238 | Critical magnetic field of superconductivity destruction | |
| 239 | Prohibited area | 7.8 eV |
| 240 | Charge carrier concentration | |
| 241 | Mohs hardness | 5,5-6 |
| 242 | Brinell hardness | |
| 243 | Vickers hardness | |
| 244 | Sound speed | |
| 245 | Surface tension | |
| 246 | Dynamic viscosity of gases and liquids | |
| 246 | Explosive concentrations of gas-air mixture, % volume | |
| 247 | Explosive concentrations of a mixture of gas and oxygen, % volume | |
| 248 | Ultimate tensile strength | |
| 249 | Yield strength | |
| 250 | Elongation limit | |
| 251 | Young's modulus | |
| 252 | Shear modulus | |
| 253 | Bulk modulus of elasticity | |
| 254 | Poisson's ratio | |
| 255 | Refractive index | 1.7355 (under normal conditions for line D, whose wavelength is approximately 0.5893 μ) |
Magnesium oxide digestibility. The best magnesium supplement. Composition of drugs
Let's decide on the goal. We decided to choose the best magnesium preparation. We will not invent anything new. We will use the logic that is involved in choosing any technique.
What often falls into the “best” category for us? Most often it is value for money. We don't like to overpay for a name or label, but we also don't like to throw money away by buying a dubious product at a low price. A stingy person pays twice (and in the case of health, he may not pay).
So, we want to please our body with magnesium.
Organic salts are good due to better bioavailability and additional effects on the body.
First, let's introduce the most common forms, where magnesium is hidden in organic compounds (organic life forms will agree with me), and then inorganic sources (silicate life forms will be accused of racism).
When choosing the best magnesium preparation, we take into account the properties of the salts:
- Magnesium citrate. Citrate.
- Magnesium malate. Malic acid salt.
- Aspartate or magnesium aspartate. Salt of aspartic (aminosuccinic) acid.
- Magnesium orotate. Orotic acid salt.
- Magnesium lactate. Salt of lactic acid.
| Substance name | Value and role for the body |
| Magnesium citrate | Citric acid is the main intermediate product of the tricarboxylic acid metabolic cycle. Plays an important role in the system of biochemical reactions of cellular respiration. In an aqueous solution, it forms chelate complexes with ions of calcium, magnesium, copper, iron, etc. When taken orally in small doses, it activates the Krebs cycle, which helps accelerate metabolism. The bioavailability of citrates is high. |
| Magnesium malate | Malic acid is an intermediate product of the tricarboxylic acid cycle and the glyoxylate cycle. That is, it is an essential substance for cellular respiration and metabolism. Malic acid is found in unripe apples, grapes, rowan, barberry, raspberries, etc. The bioavailability of malates is high. |
| Aspartate (magnesium aspartate) | Aminosuccinic acid is one of the 20 proteinogenic amino acids in the body. Plays an important role in the metabolism of nitrogenous substances, participates in the formation of pyrimidine bases and urea. Aspartic acid and asparagine are critical for the growth and proliferation of leukemia cells in some types of lymphocytic leukemia. Good bioavailability. |
| Magnesium orotate | Orotic acid is a vitamin-like substance that affects metabolism and stimulates the growth of living organisms, but does not have all the properties characteristic of vitamins. It is synthesized in sufficient quantities (cases of hypovitaminosis have not yet been described in the literature). Bioavailability is good. |
| Magnesium lactate | Lactic acid is formed during the breakdown of glucose. Sometimes called “blood sugar,” glucose is the main source of carbohydrates in our body. In the food industry it is used as a preservative, food additive E270. PLA plastic is produced by polycondensation of lactic acid. Bioavailability is good. |
| Magnesium sulfate | Inorganic substance. Used as a saline laxative when taken orally. In a hospital setting - intravenous administration. Not suitable for replenishing magnesium deficiency. |
| Magnesium oxide | Inorganic substance. It is practically insoluble in a neutral environment. In terms of bioavailability, it is tens of times inferior to organic analogues. Copes well with the task of combating constipation. |
Precautionary measures
The reagent is low-toxic, but can cause irritation upon direct contact with the eyes and respiratory organs; ingestion may cause intestinal upset. Allergic reactions are possible in case of individual intolerance.
The workplace for working with magnesium oxide should be located in a room with a forced ventilation system. Employees must use masks or respirators, goggles for respiratory and eye protection, and rubber gloves. Smoking is prohibited in the workplace. The reagent is packaged and transported in hermetically sealed plastic or multi-layer paper bags. The integrity of the packaging should be strictly monitored, as the product actively absorbs moisture from the environment.
Magnesia is stored in dry, well-ventilated warehouses, protected from direct sunlight. The substance should be placed away from heating devices, acids, and halogens. In laboratories it is stored in airtight glass or plastic containers with a ground or screw cap.
Crystal lattice:
| 300 | Crystal cell | |
| 311 | Crystal grid #1 | |
| 312 | Lattice structure | Cubic face centered |
| 313 | Lattice parameters | 0.4212 nm |
| 314 | c/a ratio | |
| 315 | Debye temperature | |
| 316 | Name of space symmetry group | Fm_3m |
| 317 | Symmetry space group number | 225 |
Magnesium oxide, what kind of oxide. Niche uses
MgO is one of the components in Portland cement.
Magnesium oxide is widely used in soil and underground rehabilitation, wastewater treatment, drinking water treatment, air emission treatment, and wastewater treatment industry for its acid buffering capacity and associated effectiveness in stabilizing dissolved heavy metal species.
Many types of heavy metals such as lead and cadmium are most soluble in water at acidic pH (below 6) as well as high pH (above 11). Metal solubility influences species bioavailability and mobility in soil and subsurface systems. Most types of metals are toxic to humans at certain concentrations, so it is critical to minimize metal bioavailability and mobility.
Granular MgO is often mixed into metal-contaminated soils or waste material, which also typically has a low (acidic) pH, in order to control the pH in the 8-10 range, where most metals are at their lowest solubility. Metal-hydroxide complexes tend to precipitate from aqueous solution in the pH range 8-10. MgO is widely regarded as the most effective stabilization of metal compounds compared to Portland cement, lime, kiln dust products, waste power generation products, and various proprietary products due to MgO's superior buffering capacity, cost effectiveness, and ease/safety of handling .
Most, if not all, products that are marketed as metal stabilization technologies create very high pH conditions in aquifers, whereas MgO creates ideal aquifer conditions with a pH of 8-10. In addition, magnesium, an essential element for most biological systems, is provided to soil and groundwater microbial populations through the MgO process, with metal-assisted rehabilitation as an added benefit.