pharmachologic effect
Oral hypoglycemic drug. Stimulates the release of insulin from functioning pancreatic β-cells. Blocks ATP-dependent channels in β-cell membranes through target proteins, which leads to depolarization of β-cells and the opening of calcium channels. An increased influx of calcium ions induces insulin secretion. In patients with type 2 diabetes mellitus, an insulinotropic response to food intake is observed within 30 minutes after ingestion. This ensures a decrease in blood glucose concentrations throughout the entire meal period. At the same time, the concentration of repaglinide in plasma decreases rapidly, and 4 hours after taking the drug, low concentrations of repaglinide are found in the plasma of patients with type 2 diabetes mellitus. When repaglinide is used in a dose range from 0.5 to 4 mg, a dose-dependent decrease in glucose concentration is observed.
Diaglinid®
It is necessary to take into account the possible interaction of repaglinide with drugs that affect glucose metabolism.
The metabolism and thus the clearance of repaglinide may be altered by drugs that act by inhibiting or activating cytochrome P450 enzymes.
Particular caution should be exercised when co-administering inhibitors of CYP2C8 and CYP3A4 with repaglinide. Studies have shown that the simultaneous administration of deferasirox, which is a weak inhibitor of CYP2C8 and CYP3A4, and repaglinide leads to an increase in the systemic exposure of repaglinide; in this case, a small but significant decrease in blood glucose concentration occurs. If deferasirox and repaglinide are co-administered, consider reducing the dose of repaglinide and carefully monitor blood glucose concentrations.
When clopidogrel, a CYP2C8 inhibitor, was co-administered with repaglinide, an increase in the systemic exposure of repaglinide and a slight decrease in blood glucose concentrations were observed. If repaglinide and clopidogrel are used concomitantly, close monitoring of glucose concentrations and clinical observation should be performed.
Inhibitors of the anion transport protein OATP1B1 (eg, cyclosporine) may also increase plasma concentrations of repaglinide.
The following drugs may enhance and/or prolong the hypoglycemic effect of repaglinide
:
Gemfibrozil, trimethoprim, rifampicin, clarithromycin, ketoconazole, itraconazole, cyclosporine, other hypoglycemic drugs, monoamine oxidase inhibitors, non-selective beta blockers, angiotensin-converting enzyme inhibitors, salicylates, non-steroidal anti-inflammatory drugs (NSAIDs), octreotide, ethanol and anabolic steroids.
Beta blockers may mask the symptoms of hypoglycemia.
Co-administration of cimetidine, nifedipine or simvastatin (which are CYP3A4 substrates) with repaglinide does not have a significant effect on the pharmacokinetic parameters of repaglinide.
Repaglinide does not have a clinically significant effect on the pharmacokinetic properties of digoxin, theophylline or warfarin when used in healthy volunteers. Thus, there is no need for dose adjustment of these drugs when used together with repaglinide.
The following drugs may reduce the hypoglycemic effect of repaglinide:
Oral contraceptives, rifampicin, barbiturates, carbamazepine, thiazides, glucocorticosteroids, danazol, thyroid hormones and sympathomimetics.
Concomitant use of oral contraceptives (ethinyl estradiol/levonorgestrel) does not lead to a clinically significant change in the overall bioavailability of repaglinide, although the maximum concentration of repaglinide is achieved earlier.
Repaglinide does not have a clinically significant effect on the bioavailability of levonorgestrel, but its effect on the bioavailability of ethinyl estradiol cannot be excluded.
In this regard, during the period of prescribing or discontinuing the above drugs, patients already receiving repaglinide should be closely monitored for timely detection of violations of glycemic control.
Contraindications
type 1 diabetes mellitus; diabetic ketoacidosis; diabetic precoma and coma; infectious diseases, major surgical interventions and other conditions requiring insulin therapy; severe liver dysfunction; simultaneous administration of gemfibrozil; lactase deficiency, lactose intolerance, glucose-galactose malabsorption; pregnancy; breastfeeding period; age under 18 years; known hypersensitivity to repaglinide or to any of the components of the drug.
Clinical studies have not been conducted in patients under 18 years of age and over 75 years of age.
It should be used with caution (the need for more careful monitoring) in case of mild to moderate liver dysfunction, febrile syndrome, chronic renal failure, alcoholism, general serious condition, malnutrition.
Dosage regimen
Diaglinide is prescribed as an addition to diet and exercise to reduce blood glucose concentrations; its administration should be timed with meals.
The drug is taken orally before main meals, usually 15 minutes before the start of a meal, but can also be taken in the interval from 30 minutes before meals until the immediate moment of eating.
The dose of the drug is selected individually for each patient depending on the concentration of glucose in the blood.
The initial dose is 0.5 mg/day, if the patient was taking another oral hypoglycemic drug - 1 mg. Dose adjustment is carried out once a week or once every 2 weeks (at the same time, they are guided by the concentration of glucose in the blood as an indicator of response to therapy). The average daily dose is 4 mg 3 times a day; maximum - 16 mg/day.
Transferring patients from therapy with other oral hypoglycemic drugs to therapy with repaglinide can be done immediately. However, the exact relationship between the dose of repaglinide and the dose of other hypoglycemic drugs has not been identified. The recommended maximum starting dose of repaglinide when switching from other hypoglycemic drugs is 1 mg before the main meal.
Combination therapy
Repaglinide may be prescribed in combination with metformin or thiazolidinediones in cases of inadequate blood glucose control on metformin, thiazolidinediones or repaglinide monotherapy. In this case, the same initial dose of repaglinide is used as with monotherapy. Then the dose of each drug is adjusted depending on the achieved blood glucose concentration.
Special patient groups
It is not recommended to prescribe repaglinide to persons under 18 years of age due to the lack of sufficient data on its safety and effectiveness in this group of patients.
Repaglinide: possibilities for clinical use in patients with type 2 diabetes mellitus
The article describes the mechanisms of the pathogenetic action of drugs from the meglitinide group using the example of nateglinide and repaglinide. It has been shown that the greatest therapeutic results from the use of these insulin secretagogues are achieved in the early stages of diabetes, when the mass and secretory potential of beta cells are partially preserved. Based on data from international clinical studies, it has been proven that repaglinide has high hypoglycemic efficacy, as well as a safety profile comparable to sulfonylureas (SMU) in relation to hypoglycemia and a higher safety profile in relation to the effect on body weight compared to PSM, glinides and insulin preparations. Repaglinide (Diaglinide produced by AKRIKHIN OJSC) can be recommended as monotherapy or in combination with metformin, basal insulin therapy at all stages of type 2 diabetes.
Rice. 1. Insulin secretion and its effect on metabolism
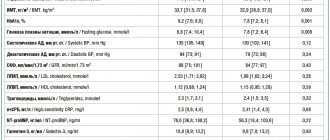
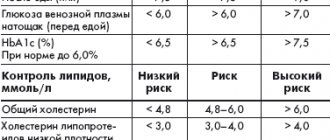
Table 1. Treatment goals for type 2 diabetes (EASD/ADA, 2009; ADA, 2012; ESC, 2009; ESC, 2011)
Rice. 2. Mean plasma insulin levels (± SEM) after administration of nateglinide 120 mg, repaglinide 0.5 and 2 mg, and placebo 10 minutes before meals 80 70 60 50 40 30 *† 20 nateglinide 120 mg Repaglinide 2 mg Repaglinide 0.5 mg Placebo *† * *‡ * *
Rice. 3. Mean plasma insulin concentrations (± SEM) at time intervals after administration of 120 mg nateglinide, 0.5 and 2 mg repaglinide, and placebo 10 minutes before meals
Rice. 4. Mean plasma glucose concentration (±SEM) after administration of 120 mg nateglinide, 0.5 and 2 mg repaglinide, and placebo 10 minutes before meals
Rice. 5. Mean plasma glucose concentration (± SEM) at time intervals after administration of 120 mg nateglinide, 0.5 and 2 mg repaglinide, and placebo 10 minutes before meals
Diabetes mellitus (DM) type 2 is one of the most important problems of modern healthcare. The prevalence of this disease throughout the world increases exponentially every year. The relevance of treatment of type 2 diabetes is determined by high rates of morbidity, disability and mortality as a result of micro- and macrovascular complications of diabetes.
According to the American Diabetes Association (2012), type 2 diabetes is a group of metabolic disorders characterized by progressive hyperglycemia, which results from impaired insulin secretion at the level of pancreatic beta cells, severe insulin resistance, or a combination of both. Type 2 diabetes is characterized by a combination with obesity, arterial hypertension, and dyslipidemia, which is a powerful predictor of cardiovascular diseases and metabolic syndrome.
Despite the fact that insulin resistance is considered the earliest and most significant pathogenetic factor of diabetes, it should be noted that hyperglycemia can develop only if there is pathology at the level of the islet apparatus of the pancreas. It is the morphological and/or functional defect of its beta cells that determines the severity and progression of hyperglycemia, and, consequently, the possibility of correcting this serious disease.
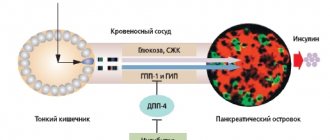
It is known that the adult pancreas secretes about 35–50 IU of insulin per day (0.6–1.2 IU/kg body weight per day), necessary to maintain normal carbohydrate metabolism. There are basal and stimulated insulin secretion. Basal insulin secretion occurs at a rate of approximately 1 U/h (24 U/day) and is responsible for reducing hepatic glucose production, maintaining a normal fasting glycemic range, and maintaining physiological levels of free fatty acids (FFAs). Stimulated (food) insulin secretion accounts for at least 50–60% of daily production and ensures regulation of blood glucose concentrations after meals. The production of stimulated insulin largely depends on the amount of carbohydrates eaten and is normally characterized by two phases - early (first, acute) and delayed (second). The early peak of insulin secretion is of greatest importance. It is this process that ensures the suppression of glucagon secretion and glucose production by the liver, increasing the peripheral sensitivity of target tissues to the action of insulin and suppressing lipolysis (Fig. 1).
In type 2 diabetes, numerous complex disorders of insulin secretion are observed at the level of pancreatic beta cells, which impairs the utilization of glucose and its use as a priority energy substrate. According to the prospective study UKPDS (United Kingdom Prospective Diabetes Study), already at the time of manifestation of type 2 diabetes, insulin secretion can be reduced by an average of 50%, and subsequently beta cell function decreases by approximately 4–6% per year, depending on degree of compensation [1]. Along with a true decrease in the number of functioning beta cells due to their accelerated apoptosis, an extremely significant factor in reducing insulin secretion is the loss of sensitivity of these cells to glucose, as well as a violation of the pulsatile nature of secretion.
The mechanisms responsible for the decrease in insulin secretory function of beta cells have not been studied, but the progressive nature of this disorder has been proven, regardless of the type of hypoglycemic effect (diet therapy, oral hypoglycemic drugs (OHDs), insulin therapy).
In modern endocrinology, the main causes of impaired secretory activity of beta cells include both genetic and environmental factors, as well as metabolic disorders (pathogenetic lipotoxicity, glucose toxicity, amyloidosis).
Functional inferiority of beta cells of the islet apparatus of the pancreas is an independent powerful factor in the development and subsequent progression of type 2 diabetes. That is why medicinal technologies aimed at restoring its secretory functions are a priority in the treatment of the disease. The main goal of therapy for type 2 diabetes is to achieve blood plasma glucose levels as close to normal as possible, provided there is no risk of developing hypoglycemia.
The modern approach to the treatment of type 2 diabetes involves individualizing the goals and methods of treating the disease depending on the patient’s age, the presence of concomitant cardiovascular pathology, the severity of diabetic complications, and the patient’s life expectancy. Equally important is reducing the level of HbA1c (glycated hemoglobin) to a certain target value [2]. It should be noted that an HbA1c level of 7% or higher should be a signal for correction/intensification of therapy to reduce micro- and macrovascular complications and improve the long-term prognosis of diabetes.
When treating type 2 diabetes, the following must be considered:
- the risk of developing complications of diabetes depends on the speed of compensation and the level of glycemia achieved and does not depend on the treatment method;
- intensive glycemic control compared with traditional treatment can increase the frequency of hypoglycemia, while not affecting the level of general and cardiovascular mortality due to diabetes, but at the same time helps reduce the risk of microvascular complications;
- HbA1c is the main integral indicator of glycemic control;
- To minimize the risk of microvascular and neuropathic complications in most patients, an HbA1c level of 6.5–7% should be achieved;
- in patients with a tendency to hypoglycemia (especially severe), short life expectancy, severe macrovascular complications or severe comorbidities, a target HbA1c level of 7–8% is allowed;
- in patients with stable control of diabetes and achieved glycemic targets, it is advisable to assess HbA1c levels at least once every 6 months, and if there is a change in treatment or the glycemic target is not achieved - once every 3 months;
- postprandial glycemia should be considered as an independent (along with HbA1c level) most important indicator of target glycemic control, which determines the development of diabetic retinopathy, atherosclerosis, the risk of myocardial infarction and death.
In addition to normalizing carbohydrate metabolism, important components of the treatment of diabetes are lowering blood pressure (BP) to a level of less than 130/80 mmHg.
Art. and normalization of blood lipid levels (Table 1). Treatment of type 2 diabetes includes the following mandatory components:
- changing the patient's lifestyle (diet, exercise, giving up bad habits -
- smoking, drinking alcohol);
- control of all modifiable risk factors;
- taking antihyperglycemic drugs from the onset of the disease;
- timely combination therapy of PSP, insulin therapy.
- The success of diabetes treatment largely depends on the patient’s compliance, the degree of his motivation, and awareness of the characteristics of his disease. In most patients, the use of oral hypoglycemic drugs is preferable to injectable forms of drugs. In this regard, drugs of the pharmacological group meglitinides (repaglinide), which affect fasting glycemia, postprandial glycemia and HbA1c, deserve special attention. This group of PSPs is used quite widely in the world, but still significantly less frequently than drugs of the sulfonylurea class (SMU).
“New” secretagogues – meglitinides – predominantly have a stimulating effect on beta-cell insulin secretion.
Repaglinide, a drug from the meglitinide group with a faster onset and shorter duration of action than PSM, is a derivative of carbamoyl-methyl-benzoic acid. It stimulates insulin secretion by binding to its own specific site with a molecular weight of 36 kDa, which is part of the ATP-dependent potassium channel, which determines its specific pharmacological properties. The pharmacological effect of repaglinide begins 5–10 minutes after its administration, which allows the patient to take the drug immediately before meals. The peak concentration of repaglinide in plasma is reached after 40 minutes - 1 hour, which allows you to regulate the level of postprandial glycemia. Repaglinide in vitro (unlike PSM) does not stimulate insulin secretion by beta cells in the absence of glucose in the medium, but at a glucose concentration of more than 5 mmol/l it is several times more active than PSM. The drug is quickly inactivated (half-life 40 minutes - 1 hour), which brings the level of insulin secretion after meals closer to the physiological profile, without causing persistent hyperinsulinemia. Another important property of repaglinide is the absence of a direct effect on exocytosis and suppression of insulin biosynthesis in beta cells [3]. The insulin secretory activity of meglitinides determines the power of the hypoglycemic effect, the ability to achieve target compensation for diabetes in a short period of time, while ensuring the safety of the drugs. Stimulated insulin levels return to baseline 3 hours after taking the drug, which reduces the likelihood of hypoglycemic episodes. No cases of hypoglycemic coma have been reported with the use of repaglinide. Gentle stimulation of insulin secretion is also important from the point of view of slowing down the depletion of secretory activity of beta cells and the rapid progression of diabetes, as well as the development of cardiovascular pathology.
To assess the characteristics of the insulin secretory activity of various drugs of the meglitinide group, studies were conducted that showed that taking drugs of the meglitinide group led to a significant increase in plasma insulin concentration after meals compared to placebo. During the 30-minute postprandial interval (10–40 minutes post-dose), nateglinide 120 mg produced the most rapid increase in insulin concentration compared with 2 and 0.5 mg repaglinide and placebo (Fig. 2), with a mean at a rate of 2.3, 1.3, 1.15 and 0.8 µU/ml/min, respectively. Plasma insulin concentrations at 10 and 15 minutes after nateglinide administration were significantly higher (p 2 mg (105.4 ± 19.6 μU/ml) and 0.5 mg (78.7 ± 17.1 μU /ml) repaglinide. Upon reaching peak values, insulin concentrations decreased rapidly with nateglinide and after 2 hours were identical to those with placebo. At 1 hour after taking 2 mg repaglinide, the mean plasma insulin concentration was significantly higher than with nateglinide at any time. time point (p
The rapid onset of action of nateglinide resulted in higher mean plasma insulin levels at 0–0.5 hours and 0–0.75 hours after dosing compared with 0.5 mg (p 6 μU/mL) (p
The stimulatory effect of meglitinides on insulin secretion in response to exercise/carbohydrate intake is extremely important because drugs in this group reduce postprandial glycemic levels, improving metabolic control. The level of blood glucose after a meal is inversely proportional to the peripheral utilization of glucose by target tissues and is the most important component of the glycemic triad responsible for the development of long-term complications of type 2 diabetes. Such serious attention paid to postprandial glucose levels is due to the fact that a person spends most of his life in the postprandial state. The pathophysiological consequences of postprandial glycemia determine the risk of developing micro- and macrovascular diabetic disorders and progression of atherosclerosis. Acute postprandial glycemia causes activation of the blood coagulation system, platelet function, and increases the activity of the renin-angiotensin-aldosterone system. Under conditions of persistent hyperglycemia, quantitative and qualitative changes in the lipid profile of an atherogenic nature are formed, the production of protein kinase C increases, vasoconstrictor factors are activated, the biological activity of NO decreases, the process of cellular oxidative stress is launched, NF-κB receptors are activated and, as a consequence, the development of endothelial dysfunction and atherogenesis.
The study results showed that mean plasma glucose levels increased rapidly after breakfast and peaked 45 minutes after administration of each meglitinide drug studied (Figure 4). Peak glucose concentrations were lower after nateglinide compared with repaglinide at both doses and placebo (p
The mean glucose level in the interval 0-0.75 hours was lower after taking nateglinide compared with other drugs (p In the interval 2-8 hours after taking nateglinide, the mean glucose level was identical to that with placebo, while with repaglinide A prolonged hypoglycemic effect was observed at both dosages compared to placebo (p
A significant decrease in postprandial glycemia proves the critical role of the early phase of insulin secretion, which improves with meglitinides. Both drugs effectively reduced glycemic levels within two hours after administration, causing high-amplitude insulin secretion. However, with repaglinide, recovery of initial glycemic parameters occurred more slowly. The relatively slow onset and long duration of action of repaglinide naturally led to more pronounced dynamics of postprandial glycemia and hyperinsulinemia. The initial pharmacokinetic profiles of nateglinide and repaglinide do not differ, and both drugs have a short half-life in the bloodstream (1–1.5 hours). In contrast to the significantly shorter half-life of repaglinide in plasma, prolonged stimulation of insulin secretion after its administration indicates a longer pharmacodynamic effect due to a prolonged receptor response [4].
Inactivation of repaglinide occurs in the liver, more than 90% of it is excreted in the bile, which allows the drug to be used in patients not only in the late stages of diabetic kidney damage, but also with concomitant renal pathology of a different origin.
Repaglinide should be taken at a dose of 0.5–4 mg before main meals 2–4 times a day. A convenient dosage and regimen of taking the drug allow the patient to comply with the regimen and frequency of meals. If you skip a meal (for example, lunch), the drug is also skipped. This regimen of taking the drug creates a great advantage both for young patients leading an active lifestyle and for older people, since the quality of life does not decrease and the risk of hypoglycemia does not increase. The maximum dose of repaglinide can be 16 mg per day, however, current recommendations emphasize the need for a timely transition to combination therapy and the unjustified use of maximum doses of any PSP. Repaglinide as monotherapy can be recommended to patients with type 2 diabetes as an alternative to metformin in case of intolerance or contraindications. In the early stages of diabetes, when the mass and secretory potential of beta cells are partially preserved, the use of repaglinide in patients with type 2 diabetes can provide a good therapeutic result. The possibilities of prandial regulation during the use of repaglinide should be especially emphasized, which suggests its effectiveness in combination therapy. Repaglinide can be combined with metformin or basal insulin therapy and is recommended at all stages of intensification of glucose-lowering therapy.
In Russia, the original drug of repaglinide is available - NovoNorm, produced by Russia, and since 2011, there is also a drug produced in Russia, registered under the name Diaglinide. Bioequivalence studies of the domestic drug have proven the high efficiency and safety of Diaglinide, which is as close as possible to the original drug. The values of the pharmacokinetic parameters of repaglinide, obtained from a comparative study of the tested and original drugs, were consistent with the data in the literature and did not differ significantly from each other. The maximum concentration of repaglinide in the blood plasma of healthy volunteers was achieved after taking Diaglinide after an average of 0.95 ± 0.69 hours, and NovoNorma after 1.35 ± 1.02 hours and amounted to 32.5 ± 20.2 ng/ml and 31 .1 ± 11.7 ng/ml, respectively. The average values of the area under the pharmacokinetic curve AUC0-t when taking the test and reference drugs also had similar values: 46.4 ± 24.4 ng/h/ml and 46.1 ± 16.6 ng/h/ml, respectively. The degree of relative bioavailability of repaglinide when taking Diaglinide tablets in relation to NovoNorm averaged 103.4 ± 43.2% (CI (confidence interval) 87.1–101.9%). Adverse events with a single dose of Diaglinide and NovoNorma tablets were similar and were classified as manifestations of the main pharmacodynamic effect [5].
Despite the fact that in Russian, European and American algorithms for the treatment of type 2 diabetes meglitinides are classified as reserve drugs, according to international experts, repaglinide should be considered as an appropriate or even preferable therapy in special situations or in certain groups of patients. The results of numerous randomized placebo-controlled trials and safety reports prove:
- high hypoglycemic efficacy of repaglinide, confirmed by reliable positive dynamics of basal, postprandial glycemia and HbA1c levels at different stages of diabetes, both in monotherapy and in combination with drugs of other pharmacological groups;
- repaglinide has similar efficacy to PSM, metformin or glitazones when used both in monotherapy and as part of any combination. A number of studies have demonstrated the superiority of repaglinide over these drugs in terms of effects on postprandial glycemia;
- During therapy with repaglinide, a comparable or fewer number of episodes of hypoglycemia develops compared to PSM, repaglinide has a higher safety profile with respect to weight gain than PSM, glitazones or insulins;
- repaglinide has not been studied in the context of its effect on overall mortality, cardiovascular morbidity and mortality from cardiovascular causes, but its positive effect on the intima-media thickness of the vascular wall, markers of chronic nonspecific inflammation, lipid parameters, thrombotic aggregation, endothelial dysfunction, oxidative stress, has been proven. adiponectin level;
Repaglinide can be considered as an alternative to metformin in monotherapy and in combination with it in the following cases:
- if flexible dose titration is required (in elderly patients, during religious fasts, etc.);
- if the patient is prone to overeating or if it is necessary to correct predominantly postprandial glycemia;
- with concomitant kidney pathology of diabetic and other origins.
Thus, repaglinide can be used to manage type 2 diabetes at different stages of the disease, in any age groups, in the absence or presence of diabetic complications.
Side effect
The most common side effect is hypoglycemia, the frequency of which depends, as with any type of diabetes therapy, on individual factors such as dietary habits, drug dose, exercise and stress. The following are side effects that have been observed with repaglinide and other oral hypoglycemic agents. All side effects are divided into groups according to the frequency of development: often (≥ 1/100 to <1/10); uncommon (≥ 1/1000 to <1/100); rare (≥ 1/10,000 to <1/1000); very rare (<1/10,000) and unknown (cannot be estimated from available data).
From the immune system: very rarely - generalized hypersensitivity reactions or immunological reactions such as vasculitis may be detected; unknown - hypersensitivity reactions such as itching, rash, urticaria.
Metabolism: often - hypoglycemia; unknown - hypoglycemic coma, hypoglycemia with loss of consciousness. As with the use of other hypoglycemic agents, hypoglycemia may develop when using repaglinide. These reactions are in most cases mild and can be managed by eating carbohydrates. Severe reactions may require medical attention, including IV dextrose (glucose). The risk of hypoglycemia may increase when repaglinide interacts with other drugs.
From the organ of vision: very rarely - visual disturbances. Changes in blood glucose concentrations can lead to visual disturbances, especially at the initial stage of therapy with hypoglycemic drugs. However, these changes are usually transitory.
From the cardiovascular system: rarely - cardiovascular diseases. The risk of cardiovascular disease increases with type 2 diabetes. An increased risk of acute coronary syndrome was found in patients receiving repaglinide compared with patients receiving a sulfonylurea, but not compared with patients receiving metformin or acarbose. However, a cause-and-effect relationship has not been established.
From the digestive system: often - abdominal pain, diarrhea; very rarely - vomiting, constipation; unknown - nausea.
From the liver and biliary tract: very rarely - liver dysfunction; in very rare cases, severe liver dysfunction has been reported (a causal relationship with repaglinide has not been established), increased activity of liver enzymes.
Diaglinide
The most common side effect is hypoglycemia, the frequency of which depends, as with any type of diabetes therapy, on individual factors such as dietary habits, drug dose, exercise and stress.
The following are side effects that have been observed with repaglinide and other oral hypoglycemic agents. All side effects are divided into groups according to the frequency of development: often (>1/100 to 1/1000 to 1/10,000 to
From the immune system: very rarely - generalized hypersensitivity reactions or immunological reactions such as vasculitis may be detected; unknown - hypersensitivity reactions such as itching, rash, urticaria.
Metabolism: often - hypoglycemia; unknown - hypoglycemic coma, hypoglycemia with loss of consciousness. As with the use of other hypoglycemic agents, hypoglycemia may develop when using repaglinide. These reactions are in most cases mild and can be managed by eating carbohydrates. Severe reactions may require medical attention, including intravenous dextrose (glucose). The risk of hypoglycemia may increase when repaglinide interacts with other drugs.
From the organ of vision: very rarely - visual disturbances. Changes in blood glucose concentrations can lead to visual disturbances, especially at the initial stage of therapy with hypoglycemic drugs. However, these changes are usually transitory.
From the cardiovascular system: rarely - cardiovascular diseases. The risk of cardiovascular disease increases with type 2 diabetes. An increased risk of acute coronary syndrome was found in patients receiving repaglinide compared with patients receiving a sulfonylurea, but not compared with patients receiving metformin or acarbose. However, a cause-and-effect relationship has not been established.
From the digestive system: often - abdominal pain, diarrhea; very rarely - vomiting, constipation; unknown - nausea.
From the liver and biliary tract: very rarely - liver dysfunction; in very rare cases, severe liver dysfunction has been reported (a causal relationship with repaglinide has not been established), increased activity of liver enzymes.
In case of an overdose, hypoglycemia may develop, manifested by symptoms such as hunger, increased sweating, palpitations, tremor, anxiety, headache, insomnia, irritability, depression, speech and vision impairment.
When repaglinide was used in patients with type 2 diabetes mellitus at a weekly increasing dose from 4 to 20 mg 4 times a day (with each meal) for 6 weeks, a relative overdose was observed, manifested by an excessive decrease in glucose concentration with the development of symptoms of hypoglycemia.
If symptoms of hypoglycemia appear, appropriate measures should be taken to increase the concentration of glucose in the blood (take dextrose or foods rich in carbohydrates). For severe hypoglycemia (loss of consciousness, coma), dextrose is administered intravenously. After regaining consciousness, take easily digestible carbohydrates (to avoid re-development of hypoglycemia).
special instructions
Repaglinide is indicated for unsatisfactory glycemic control and persistence of symptoms of diabetes mellitus during diet therapy and exercise.
Because repaglinide is an insulin secretagogue, it may cause hypoglycemia. With combination therapy, the risk of hypoglycemia increases.
Major surgical interventions and injuries, extensive burns, infectious diseases with febrile syndrome may require the abolition of oral hypoglycemic drugs and the prescription of insulin.
It is necessary to regularly monitor the concentration of glucose in the blood on an empty stomach and after meals.
The patient should be warned about the increased risk of hypoglycemia when taking alcohol, NSAIDs, or fasting. Dose adjustment is necessary in case of physical and emotional stress, or changes in diet.
In malnourished and malnourished patients, care must be taken when selecting the initial and maintenance dose and its titration to avoid hypoglycemia.
Dose selection in patients with type 2 diabetes in combination with severe renal impairment should be done with caution.
Administration of normal doses of repaglinide to patients with impaired hepatic function may result in higher concentrations of repaglinide and its metabolites than in patients with normal hepatic function. In this regard, the use of repaglinide is contraindicated in patients with severe hepatic impairment, and in patients with mild to moderate hepatic impairment, repaglinide should be used with caution. The intervals between dose adjustments should also be increased to more accurately assess the response to therapy.
Diaglinide®
Repaglinide is indicated for unsatisfactory glycemic control and persistence of symptoms of diabetes mellitus during diet therapy and exercise.
Because repaglinide is an insulin secretagogue, it may cause hypoglycemia. With combination therapy, the risk of hypoglycemia increases.
Major surgical interventions and injuries, extensive burns, infectious diseases with febrile syndrome may require the abolition of oral hypoglycemic drugs and the prescription of insulin.
It is necessary to regularly monitor the concentration of glucose in the blood on an empty stomach and after meals.
The patient should be warned about the increased risk of hypoglycemia when taking alcohol, NSAIDs, or fasting. Dose adjustment is necessary in case of physical and emotional stress, or changes in diet.
In malnourished and malnourished patients, care must be taken when selecting the initial and maintenance dose and its titration to avoid hypoglycemia.
Dose selection in patients with type 2 diabetes in combination with severe renal impairment should be done with caution.
Administration of normal doses of repaglinide to patients with impaired hepatic function may result in higher concentrations of repaglinide and its metabolites than in patients with normal hepatic function. In this regard, the use of repaglinide is contraindicated in patients with severe hepatic impairment, and in patients with mild to moderate hepatic impairment, repaglinide should be used with caution. The intervals between dose adjustments should also be increased to more accurately assess the response to therapy.
Impact on the ability to drive vehicles and operate machinery
Patients' ability to concentrate and react quickly may be impaired during hypoglycemia and hyperglycemia, which can be dangerous in situations where this ability is especially necessary (for example, when driving or operating machinery). Patients should be advised to take measures to prevent the development of hypoglycemia and hyperglycemia when driving vehicles and operating machinery. This is especially important for patients with no or decreased severity of symptoms that are warning signs of developing hypoglycemia or who suffer from frequent episodes of hypoglycemia. In these cases, the feasibility of performing such work should be considered.
Impact on the ability to drive vehicles and machinery
Patients' ability to concentrate and react quickly may be impaired during hypoglycemia and hyperglycemia, which can be dangerous in situations where this ability is especially necessary (for example, when driving or operating machinery). Patients should be advised to take measures to prevent the development of hypoglycemia and hyperglycemia when driving vehicles and operating machinery. This is especially important for patients with no or decreased severity of symptoms that are warning signs of developing hypoglycemia or who suffer from frequent episodes of hypoglycemia. In these cases, the feasibility of performing such work should be considered.
Overdose
In case of overdose, hypoglycemia may develop. Symptoms: hunger, increased sweating, palpitations, tremors, anxiety, headache, insomnia, irritability, depression, speech and vision impairment.
When repaglinide was used in patients with type 2 diabetes mellitus at a weekly increasing dose from 4 to 20 mg 4 times a day (with each meal) for 6 weeks, a relative overdose was observed, manifested by an excessive decrease in glucose concentration with the development of symptoms of hypoglycemia.
Treatment: if symptoms of hypoglycemia appear, appropriate measures should be taken to increase the concentration of glucose in the blood (take oral dextrose or foods rich in carbohydrates). In case of severe hypoglycemia (loss of consciousness, coma), dextrose is administered intravenously. After regaining consciousness, take easily digestible carbohydrates (to avoid re-development of hypoglycemia).
Drug interactions
It is necessary to take into account the possible interaction of repaglinide with drugs that affect glucose metabolism.
Metabolism, and thus clearance of repaglinide, may be altered by drugs that act by inhibiting or activating cytochrome P450 enzymes. Particular caution should be exercised when co-administering inhibitors of CYP2C8 and CYP3A4 with repaglinide.
Inhibitors of the anion transport protein OATP1B1 (eg, cyclosporine) may also increase plasma concentrations of repaglinide.
The following drugs may enhance and/or prolong the hypoglycemic effect of repaglinide: gemfibrozil, trimethoprim, rifampicin, clarithromycin, ketoconazole, itraconazole, cyclosporine, other hypoglycemic drugs, MAO inhibitors, non-selective beta-blockers, ACE inhibitors, salicylates, NSAIDs, octreotide, ethanol and anabolic steroid.
Beta blockers may mask the symptoms of hypoglycemia.
Co-administration of cimetidine, nifedipine or simvastatin (which are CYP3A4 substrates) with repaglinide does not have a significant effect on the pharmacokinetic parameters of repaglinide.
Repaglinide does not have a clinically significant effect on the pharmacokinetic properties of digoxin, theophylline or warfarin when used in healthy volunteers. Thus, there is no need for dose adjustment of these drugs when used together with repaglinide.
The following drugs may weaken the hypoglycemic effect of repaglinide: oral contraceptives, rifampicin, barbiturates, carbamazepine, thiazides, corticosteroids, danazol, thyroid hormones and sympathomimetics.
Concomitant use of oral contraceptives (ethinyl estradiol/levonorgestrel) does not lead to a clinically significant change in the overall bioavailability of repaglinide, although the maximum concentration of repaglinide is achieved earlier. Repaglinide does not have a clinically significant effect on the bioavailability of levonorgestrel, but its effect on the bioavailability of ethinyl estradiol cannot be excluded.
In this regard, during the period of prescribing or discontinuing the above drugs, patients already receiving repaglinide should be closely monitored for timely detection of violations of glycemic control.
Diaglinide, 30 pcs., 2 mg, tablets
It is necessary to take into account the possible interaction of repaglinide with drugs that affect glucose metabolism.
Metabolism, and thus clearance of repaglinide, may be altered by drugs that act by inhibiting or activating cytochrome P-450 enzymes. Particular caution should be exercised when co-administering inhibitors of CYP2C8 and CYP3A4 with repaglinide. Studies have shown that the simultaneous administration of deferasirox, which is a weak inhibitor of CYP2C8 and CYP3A4, and repaglinide leads to an increase in the systemic exposure of repaglinide; in this case, a small but significant decrease in blood glucose concentration occurs. If deferasirox and repaglinide are co-administered, consider reducing the dose of repaglinide and carefully monitor blood glucose concentrations.
When clopidogrel, a CYP2C8 inhibitor, was co-administered with repaglinide, an increase in the systemic exposure of repaglinide and a slight decrease in blood glucose concentrations were observed. If repaglinide and clopidogrel are used concomitantly, close monitoring of glucose concentrations and clinical observation should be performed.
Inhibitors of the anion transport protein OATP1B1 (eg, cyclosporine) may also increase plasma concentrations of repaglinide.
The following drugs may enhance and/or prolong the hypoglycemic effect of repaglinide:
Gemfibrozil, trimethoprim, rifampicin, clarithromycin, ketoconazole, itraconazole, cyclosporine, other hypoglycemic drugs, monoamine oxidase inhibitors, non-selective beta blockers, angiotensin-converting enzyme inhibitors, salicylates, non-steroidal anti-inflammatory drugs (NSAIDs), octreotide, ethanol and anabolic steroids.
Beta blockers may mask the symptoms of hypoglycemia.
Co-administration of cimetidine, nifedipine or simvastatin (which are CYP3A4 substrates) with repaglinide does not have a significant effect on the pharmacokinetic parameters of repaglinide.
Repaglinide does not have a clinically significant effect on the pharmacokinetic properties of digoxin, theophylline or warfarin when used in healthy volunteers. Thus, there is no need for dose adjustment of these drugs when used together with repaglinide.
The following drugs may reduce the hypoglycemic effect of repaglinide:
Oral contraceptives, rifampicin, barbiturates, carbamazepine, thiazides, glucocorticosteroids, danazol, thyroid hormones and sympathomimetics.
Concomitant use of oral contraceptives (ethinyl estradiol/levonorgestrel)
does not lead to a clinically significant change in the overall bioavailability of repaglinide, although the maximum concentration of repaglinide is achieved earlier. Repaglinide does not have a clinically significant effect on the bioavailability of levonorgestrel, but its effect on the bioavailability of ethinyl estradiol cannot be excluded.
In this regard, during the period of prescribing or discontinuing the above drugs, patients already receiving repaglinide should be closely monitored for timely detection of violations of glycemic control.