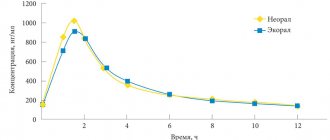
Sunitinib is an oral, multitargeted tyrosine kinase inhibitor that inhibits tumor growth, pathological angiogenesis, and metastasis. Indications for use are: gastrointestinal stromal tumors, renal cell carcinoma.
The purpose of the S-TRAC analysis was to determine the efficacy and safety profile of sunitinib in patients with high-recurrence locoregional renal cell carcinoma following nephrectomy.
Methods
The phase 3, randomized, double-blind study included 615 patients 18 years of age or older with high-risk locoregional clear cell renal cell carcinoma. Patients were randomized into 2 groups: sunitinib 50 mg daily or placebo for 4 weeks, then 2 weeks off, and then on a 4/2 schedule for one year or until disease relapse, severe drug toxicity, or the patient was excluded from the analysis.
The disease-free period was chosen as the primary endpoint; the disease-free period assessed by investigators, overall survival, and safety of therapy were selected as secondary endpoints.
Sunitinib-native instructions for use
Active substance
Sunitinib
Dosage form: Dosage 12.5 mg: hard gelatin capsules No. 4, yellow body, yellow cap. Dosage 25 mg: hard gelatin capsules No. 3, orange body, orange cap. Dosage 50 mg: hard gelatin capsules No. 2, red-brown body, red-brown cap. The contents of the capsules are powder or granules from yellow to orange.
Composition: Active substance: sunitinib malate 66.8 mg, which corresponds to the content of sunitinib 50 mg Excipients: mannitol - 79.3 mg, croscarmellose sodium - 10 mg, povidone K30 - 8.4 mg, magnesium stearate - 2.5 mg. Composition of the capsule shell: titanium dioxide - 0.5 mg, red iron oxide dye - 1.5 mg, gelatin up to 100%. Composition of the capsule cap: titanium dioxide - 0.5 mg, red iron oxide dye - 1.5 mg, gelatin up to 100%.
Pharmacotherapeutic group: Antitumor agent - protein tyrosine kinase inhibitor
ATC: L01 Antitumor drugs
Pharmacodynamics: Sunitinib is able to simultaneously inhibit the receptors of various tyrosine kinases (RTKs) involved in the processes of tumor growth, pathological angiogenesis and the formation of metastases. Shows inhibitory activity against many kinases (> 80 kinases). It has been shown to be a potent inhibitor of platelet-derived growth factor receptors (PDGFRα and PDGRFβ), vascular endothelial growth factor receptors (VEGRF1, VEGRF2 and VEGRF3), stem cell factor receptor (KIT), Fms-like tyrosine kinase-3 receptor (FLT), colony-stimulating factor receptor (CSF-1R) and glial-derived neurotrophic factor receptor (RET). The activity of the main metabolite was similar to that of sunitinib. Sunitinib inhibited the phosphorylation of multiple RTKs (PDGRFβ, VEGRF2, and KIT) in tumor xenografts expressing target RTKs in vivo and demonstrated inhibition of tumor growth or regression and/or inhibition of metastasis in experimental models of various tumors. Sunitinib has demonstrated the ability to inhibit the growth of tumor cells expressing deregulated target PTKs (PDGFR, RET, or KIT) in vitro and PDGRFβ- and VEGRF2-dependent angiogenesis in vivo.
Pharmacokinetics: Absorption Sunitinib is well absorbed when administered orally. The time to reach maximum concentration (Cmax) is 6-12 hours (Tmax) after administration. Food intake does not affect the bioavailability of sunitinib. Distribution: The binding of sunitinib and its main metabolite to plasma proteins is 95% and 90%, respectively, with no apparent concentration dependence in the range from 100 to 4000 ng/ml. The estimated volume of distribution in tissues (Vd/F) is 2230 l. Metabolism Sunitinib is metabolized primarily by CYP3A4, a cytochrome P450 enzyme, resulting in the formation of the main active metabolite, which is further metabolized by the same CYP3A4 isoenzyme. The proportion of the active metabolite is 23-37% of the area under the curve “very often” ≥10%; “common” ≥ 1% and < 10%, “infrequently” ≥0.1% and < 1%, “rare” ≥ 0.01% < 0.1%, “very rare” - < 0.01%. Disorders of the blood and lymphatic system: very often - anemia, neutropenia, thrombocytopenia; often - leukopenia, lymphopenia, decreased hemoglobin concentration; infrequently - pancytopenia. Gastrointestinal tract disorders: very often - taste disturbance, diarrhea, nausea, vomiting, stomatitis (including aphthous), mucositis, dyspepsia, abdominal pain, anorexia, constipation, glossodynia (neuralgia of the tongue), flatulence, dryness oral mucosa; often - pain in the mouth, bloating, gastroesophageal reflux, loss of appetite, bleeding gums, dysphagia, ulceration of the oral mucosa, cheilitis, pain in the anus, hemorrhoids, rectal bleeding, belching; uncommon - pancreatitis; rarely - gastrointestinal perforation. Disorders of the skin and subcutaneous tissues: very often - discoloration of the skin, palmar-plantar syndrome (erythrodysesthesia), rash (erythematous, macular, papular, pityriasis, generalized, psoriasis-like), blisters, discoloration of hair, dry skin, erythema; often - alopecia, peeling of the skin, itching, exfoliative dermatitis, impaired nail growth, yellow skin discoloration, hyperkeratosis, acne, skin hyperpigmentation, eczema; rarely - Stevens-Johnson syndrome. Musculoskeletal and connective tissue disorders: often - pain in the limbs, arthralgia, myalgia, muscle spasm, back pain, muscle weakness. Nervous system disorders: very often - headache; often - dizziness, paresthesia, insomnia or increased drowsiness, peripheral neuropathy. Cardiac disorders: often - decreased left ventricular ejection fraction (LVEF); uncommon - heart failure, including chronic, dysfunction of the left ventricle, pericardial effusion; rarely - prolongation of the QT interval, atrial fibrillation and atrial flutter of the “pirouette” type. Vascular disorders: very often - increased blood pressure; often - venous thromboembolism (pulmonary embolism, deep vein thrombosis), "flushes" of blood. Disorders of the kidneys and urinary tract: often - chromaturia (discoloration of urine); infrequently - acute renal failure. Disorders of the respiratory system, chest and mediastinal organs: very often - nosebleeds, cough; often - shortness of breath, laryngopharyngeal pain, pleural effusion, dryness of the nasal mucosa; infrequently - hemoptysis. Endocrine system disorders: often - hypothyroidism, increased concentration of thyroid-stimulating hormone. Visual disturbances: often - increased lacrimation, periorbital edema, swelling of the eyelids. Laboratory and instrumental data: very often - increased lipase activity in the blood serum; often - increased activity of liver enzymes, increased concentration of creatinine in the blood plasma, increased activity of creatine phosphokinase and amylase in the blood serum, increased concentration of uric acid in the blood plasma, decrease in body weight. General disorders and disorders at the injection site: very often - asthenia, increased fatigue; often - flu, fever, chills, peripheral edema, dehydration, chest pain; infrequently - bleeding from tumors, delayed wound healing; rarely - tumor lysis syndrome (in some cases fatal). In patients with brain metastases or reversible leukoencephalopathy syndrome, cases of seizures, in some cases with a fatal outcome, have been described. Results of post-marketing studies of sunitinib The following adverse events were recorded during the use of sunitinib after its marketing authorization: Blood and lymphatic disorders: rare cases of thrombotic microangiopathy have been reported. In such cases, it is recommended to temporarily stop taking sunitinib; After symptoms resolve, the drug may be resumed at the discretion of the attending physician. Respiratory, thoracic and mediastinal disorders: cases of pulmonary embolism, sometimes fatal, have been reported. Endocrine disorders: Rare cases of hyperthyroidism progressing to hypothyroidism have been reported in clinical trials of sunitinib and during post-marketing use of sunitinib; infrequently - thyroiditis. Immune system disorders: hypersensitivity reactions, including angioedema. Infectious and parasitic diseases: Cases of serious infections (including those associated with neutropenia), some of which resulted in death, have been reported. Infections that develop during the use of sunitinib are common in cancer patients (often respiratory infections (pneumonia, bronchitis), urinary tract infections, skin infections (for example, inflammation of the subcutaneous fatty tissue), abscess (for example, oral cavity, genital area, anorectal area, skin, extremities, visceral abscess), infrequently - sepsis/septic shock). Often, infections can be bacterial (eg, intra-abdominal, osteomyelitis), viral (eg, nasopharyngitis, oral herpes) or fungal (eg, oral candidiasis, esophageal candidiasis). Rare cases of necrotizing fasciitis, including perineal involvement, have been reported, sometimes with fatal outcome. Musculoskeletal and connective tissue disorders: There have been reports of rare cases of myopathy and/or rhabdomyolysis, with or without combination with acute renal failure, with rare cases of death. The majority of these patients had underlying risk factors and/or received concomitant therapy with drugs that are prone to adverse reactions of this kind. There are also case reports of fistula formation, sometimes associated with necrosis and/or tumor regression, some of which were fatal. Cases of necrosis of the jaw have been reported with the use of sunitinib. Most patients had risk factors for developing jaw necrosis, such as intravenous bisphosphonate use and/or previous dental treatment requiring invasive intervention. Nervous system disorders: Cases of taste disorders, including ageusia, have been reported. Renal and urinary tract disorders: There have been reports of cases of renal dysfunction/renal failure, some of which have been fatal. Cases of proteinuria and rare cases of nephrotic syndrome have been reported. Cardiac disorders: Cases of cardiomyopathy have been reported, some of which have been fatal. Vascular disorders: There have been reports of arterial thromboembolism (some fatal) in patients receiving sunitinib. The most common were: stroke, transient ischemic attack. Risk factors, in addition to the underlying disease and the patient's age over 65 years, are: arterial hypertension, diabetes mellitus, history of thromboembolic complications. Cases of bleeding, sometimes fatal, have been reported, including gastrointestinal bleeding, respiratory tract bleeding and cerebral hemorrhage. Skin and subcutaneous tissue disorders: There have been reports of rare cases of pyoderma gangrenosum, erythema multiforme, and toxic epidermal necrolysis. Gastrointestinal disorders: esophagitis. Disorders of the liver and biliary tract: hepatitis.
Overdose: In case of overdose with sunitinib, treatment is symptomatic; there is no specific antidote. If necessary, it is recommended to induce vomiting or perform gastric lavage.
Interaction: Drugs that increase the concentration of sunitinib in blood plasma Co-administration of a single dose of sunitinib with the CYP3A4 isoenzyme inhibitor, ketoconazole, increases the Cmax and AUC0-∞ of the sunitinib complex and the main active metabolite by 49% and 51%, respectively. The use of sunitinib in combination with other inhibitors of the CYP3A4 isoenzyme (for example, ritonavir, itraconazole, erythromycin, clarithromycin or grapefruit juice) may lead to increased sunitinib concentrations. Coadministration of CYP3A4 inhibitors with sunitinib should be avoided, or an alternative drug with minimal CYP3A4 inhibitory potential should be selected. If this is not possible, it will likely be necessary to reduce the daily dose of sunitinib by 12.5 mg to 37.5 mg per day for gastrointestinal stromal tumors and metastatic renal cell carcinoma and to 25 mg per day for pancreatic neuroendocrine tumors. Drugs that reduce the concentration of sunitinib in blood plasma The combined use of a single dose of sunitinib with the inducer of the CYP3A4 isoenzyme, rifampicin, reduces the Cmax and AUC0-∞ of the sunitinib complex and the main active metabolite by 23% and 46%, respectively. Use of sunitinib in combination with other inducers of the CYP3A4 isoenzyme (for example, dexamethasone, phenytoin, carbamazepine, rifampicin, phenobarbital or St. John's wort) may lead to decreased concentrations of sunitinib. Coadministration of CYP3A4 inducers with sunitinib should be avoided, or an alternative drug with minimal CYP3A4 inducing potential should be selected. If this is not possible, it will likely be necessary to increase the sunitinib dose by 12.5 mg while monitoring patient tolerance. The daily dose in this case should not exceed 87.5 mg for gastrointestinal stromal tumors and metastatic renal cell cancer and 62.5 mg for neuroendocrine tumors of the pancreas.
Special instructions: Treatment with Sunitinib-native should be carried out under the supervision of a physician experienced in working with anticancer drugs. At the beginning of each cycle of therapy with Sunitinib-native, a complete analysis of hematological parameters should be performed. Hemorrhages, tumor bleeding There have been reports of cases of bleeding, sometimes fatal, including gastrointestinal bleeding, bleeding from the respiratory tract, tumor, urinary tract and cerebral hemorrhage. These phenomena can occur unexpectedly, and in the case of tumor foci in the lungs, manifest in the form of severe or life-threatening hemoptysis or pulmonary hemorrhage. There have been reports of pulmonary hemorrhage (in some cases fatal) in patients taking sunitinib for renal cell carcinoma, gastrointestinal tumors, or non-small cell lung cancer. Important: Sunitinib is not intended for the treatment of non-small cell lung cancer. It is periodically necessary to conduct a medical examination and evaluate blood counts to early detect the first signs of bleeding and apply the necessary therapeutic measures. During concomitant therapy with anticoagulants, blood clotting parameters should be monitored. Epistaxis associated with sunitinib therapy was the most common type of bleeding in patients with solid tumors, occurring in 8% of cases. It was observed in half of the cases in patients with hemorrhagic complications. Cardiac dysfunction The relationship between receptor tyrosine kinase (RTK) inhibition and cardiac function has not been studied. It is unknown whether patients who have had a history of cardiovascular events in the last 12 months prior to treatment with sunitinib, such as myocardial infarction (including severe/unstable angina), coronary/peripheral bypass grafting, symptomatic congestive heart failure, cerebrovascular events, or transient ischemic events, or pulmonary embolism, are at greater risk of treatment-related left ventricular dysfunction. When prescribing Sunitinib-native to this category of patients, the risk/benefit ratio should be carefully assessed. During therapy with Sunitinib-native, patients should be periodically monitored for clinical signs and symptoms of chronic heart failure (CHF). LVEF is recommended to be assessed before initiation of therapy and periodically during treatment. If clinical signs of CHF appear, treatment with sunitinib should be discontinued. In the absence of clinical signs of CHF, but with LVEF less than 50% or a decrease in this indicator by more than 20% compared to the initial one (before starting therapy), it is recommended to reduce the dose of Sunitinib-native or stop taking the drug. Prolongation of the QT interval At concentrations approximately 2 times higher than therapeutic levels, sunitinib promotes prolongation of the QTcF interval (Frederick correction). The clinical significance of this effect is unclear and depends on the risk factors and susceptibility of the individual patient. Sunitinib should be used with caution in patients with a history of QT prolongation, in patients taking antiarrhythmic drugs, or in patients with underlying cardiac disease, bradycardia, or electrolyte disturbances. Caution is required and the dose of sunitinib should be reduced when concomitantly taking strong inhibitors of the CYP3A4 isoenzyme, which may increase the plasma concentration of sunitinib. ECG monitoring is recommended before starting therapy and during treatment with sunitinib. Hypertension Patients should be monitored for elevated blood pressure using standard monitoring methods. In patients with severe unresponsive hypertension, temporary discontinuation of sunitinib therapy is recommended. Therapy is resumed as soon as arterial hypertension is controlled. Thyroid dysfunction Monitoring all patients during sunitinib therapy for the development of thyroid dysfunction is recommended. Patients with signs and/or symptoms of thyroid dysfunction should undergo laboratory monitoring. Baseline laboratory testing of thyroid function is also recommended in patients with hypothyroidism or hyperthyroidism. Patients with hypothyroidism are treated according to standard medical practice before initiating sunitinib therapy. Skin and subcutaneous tissue disorders Patients should be warned that skin discoloration may occur during treatment with sunitinib due to the presence of a dye (yellow) in the drug. Hair or skin discoloration may also occur. Gastrointestinal disorders Since nausea and vomiting may occur with sunitinib, prophylactic antiemetics should be considered. If diarrhea occurs, antidiarrheals are prescribed. Pancreatic dysfunction During treatment with Sunititnib-native, it is necessary to periodically check the activity of lipase and amylase in the blood serum. If symptoms of pancreatitis are present or appear, regular medical monitoring is necessary. Symptoms of Reversible Posterior Leukoencephalopathy Patients with brain metastases, a history of seizures, and/or signs/symptoms of reversible posterior leukoencephalopathy, such as increased blood pressure, headache, lethargy, cognitive impairment, vision loss, including cortical blindness, should be monitored using standard methods. , including blood pressure control. If these symptoms appear during therapy, it is recommended to temporarily stop taking Sunitinib-native. After the symptoms disappear, treatment can be resumed at the discretion of the attending physician. Thrombotic microangiopathy If thrombotic microangiopathy occurs, temporary discontinuation of sunitinib treatment is recommended. After the symptoms disappear, treatment can be resumed at the discretion of the attending physician. Impaired renal function Background testing of renal function before initiation of treatment is recommended, as well as monitoring of renal function indicators during sunitinib therapy. The safety of sunitinib in patients with moderate to severe proteinuria has not been assessed. In patients with nephrotic syndrome, treatment with sunitinib should be discontinued. Liver impairment Cases of liver failure, sometimes fatal, have been reported during sunitinib therapy. Liver function tests (alanine aminotransferase, aspartate aminotransferase, bilirubin concentration) should be monitored before initiating sunitinib therapy, during each treatment cycle, and as clinically indicated. If grade 3 or 4 side effects on liver function develop, you should stop taking the drug. If symptoms of hepatotoxicity do not resolve, the drug should be discontinued. Hematological disorders There are reports of a decrease in the number of neutrophils of grades 3 and 4 (in 13.1% and 0.9% of cases, respectively). A decrease in platelet counts of grade 3 and 4 was noted in 4% and 0.5% of cases, respectively. The listed cases were not cumulative, were usually reversible and did not lead to discontinuation of therapy. In some cases, fatal bleeding was observed in combination with thrombocytopenia. Tumor lysis syndrome Patients with large tumor burdens (prior to initiation of sunitinib treatment) should be closely monitored as they are at greatest risk of developing tumor lysis syndrome. Delayed wound healing Cases of delayed wound healing have been reported with the use of sunitinib. If extensive surgery is necessary, a temporary suspension of the drug is recommended. There are limited data on the time after surgery after which therapy can be resumed. Therefore, the decision to restart therapy should be based on an assessment of the wound after surgery. Osteonecrosis of the jaw Cases of osteonecrosis of the jaw have been reported with the use of sunitinib. Most of the cases were observed in patients receiving intravenous bisphosphonates, the use of which is a risk factor for the development of osteonecrosis of the jaw. Caution should be exercised when using sunitinib and intravenous bisphosphonates simultaneously. In addition, invasive procedures for oral diseases are also risk factors for the development of osteonecrosis of the jaw. A dental examination of the patient should be performed before initiating sunitinib therapy. In patients taking sunitinib who have previously received intravenous bisphosphonate therapy, invasive oral procedures should be avoided if possible. Hypersensitivity reactions and angioedema If angioedema develops as a result of a hypersensitivity reaction, discontinue sunitinib therapy and institute standard treatment. Fistula formation If fistula occurs, sunitinib therapy should be discontinued. There are limited data on the use of sunitinib after fistula formation. Cardiomyopathy In post-marketing studies of sunitinib, cases of cardiac dysfunction (sometimes fatal), such as heart failure, cardiomyopathy, and myocardial dysfunction, have been reported. Based on this information, it is suggested that the use of suntinib may increase the risk of developing cardiomyopathy. Patients treated with sunitinib had no risk factors for cardiomyopathy other than exposure to sunitinib itself.
Effects on Fertility Based on the results of preclinical studies with sunitinib, it appears that sunitinib therapy may adversely affect fertility in both men and women. Reliable contraception must be used during and for at least three months after discontinuation of sunitinib therapy.
Impact on the ability to drive vehicles. Wed and fur.: There is no data on the effect of the drug Sunitinib-native on the ability to drive vehicles and machines. In the event of the development of undesirable reactions from the musculoskeletal and connective tissue, visual organs or nervous system when using the drug Sunitinib-native, you should refrain from driving vehicles and machinery, as well as from engaging in other potentially hazardous activities that require increased concentration and speed. psychomotor reactions.
Release form/dosage: Capsules, 12.5 mg, 25 mg and 50 mg.
Packaging: 7 capsules in a blister pack made of printed laminated aluminum foil and polyvinyl chloride film. 28 or 30 capsules in polyethylene terephthalate jars, sealed with polyethylene lids. 4 blister packs, 1 can along with instructions for use in a cardboard pack.
Storage conditions: In a place protected from light, at a temperature not exceeding 25 ° C. Keep out of the reach of children.
Shelf life: 3 years. Do not use after the expiration date stated on the packaging.
Conditions for dispensing from pharmacies: By prescription
Owner of the Registration Certificate: NATIVA, LLC
results
- The median disease-free survival period was 6.8 years (95% CI, 5.8 - not reached the limit) in the group of patients receiving sunitinib and 5.6 years (95% CI, 3.8-6.6) in the placebo group (hazard ratio (HR) ), 0.76; 95% CI, 0.59 to 0.98; P=0.03).
- Dose reductions due to adverse events occurred more frequently in the sunitinib group compared with the control group (34.3% vs. 2%), as did dose interruptions (46.4% vs. 13.2%) and discontinuation of the drug (28.1% vs. 2%). 5.6%).
- Grade 3 and 4 adverse events were more frequently reported in subjects receiving sunitinib (48.4% grade 3 and 12.1% grade 4 events) compared with controls (15.8% and 3.6%, respectively).
- The incidence of serious adverse events was comparable in both groups (21.9% for sunitimab and 17.1% for placebo), and no deaths were attributed to treatment toxicities.
Sunitinib-native
Pulmonary embolism (1%), thrombocytopenia (1%), tumor bleeding (0.9%), febrile neutropenia (0.4%), increased blood pressure (0.4%).
Venous thromboembolism in patients with metastatic renal cell cancer (pulmonary embolism (grade 4) and deep vein thrombosis (grade 3)) - 2%, in patients with gastrointestinal stromal tumors - 3%.
In 20% of cases: fatigue, diarrhea, nausea, stomatitis, dyspepsia (including vomiting), skin discoloration, taste disturbance, anorexia.
Frequency: very often (more than 1/10), often (more than 1/100 and less than 1/10), infrequently (more than 1/1000 and less than 1/100), rarely (more than 1/10000 and less than 1/1000), very rare (less than 1/10000).
From the hematopoietic organs: very often and often - anemia, neutropenia, thrombocytopenia; often - leukopenia.
From the digestive system: very often - taste perversion, diarrhea, nausea, vomiting, stomatitis, mucositis, dyspepsia, gastralgia; very often or often - anorexia, constipation, glossodynia (neuralgia of the tongue), flatulence, dry oral mucosa; often - pain in the mouth, gastroesophageal reflux; uncommon - pancreatitis; rarely - gastrointestinal perforation.
From the skin: very often - discoloration of the skin, palm-plantar syndrome (erythrodysesthesia), rash (erythematous, macular, papular, pityriasis-like, generalized, psoriasis-like), blisters; very often or often - change in hair color, dry skin, erythema; often - alopecia, peeling of the skin, itching, exfoliative dermatitis.
From the musculoskeletal system: often - pain in the limbs, arthralgia, myalgia.
From the nervous system: very often - headache; often - dizziness, paresthesia, insomnia or increased drowsiness, depression.
From the cardiovascular system: very often - increased blood pressure; often - decreased LV ejection fraction, venous thromboembolism (pulmonary embolism, deep vein thrombosis); infrequently - CHF, incl. congestive, impaired LV function, rarely - prolongation of the QT interval, type ventricular fibrillation and flutter.
From the urinary system: often - change in urine color.
From the respiratory system: very often or often - nosebleeds; often - shortness of breath, pain in the larynx, pharynx.
From the endocrine system: often - hypothyroidism, increased TSH concentration.
Other: very often - asthenia; very often or often - increased lipase activity; often - lacrimation, weight loss, fever, chills, peripheral edema, periorbital edema, dehydration, increased CPK and amylase; infrequently - bleeding from tumors, influenza-like syndrome.
Cases of seizures have been described in patients with brain metastases or reversible leukoencephalopathy syndrome.