Prostate cancer (PCa) today remains one of the pressing problems of the older male population. In the Russian Federation, the number of men diagnosed with prostate cancer (PCa) in 2012 was 29,082, while in 2000 11,580 were diagnosed.[1]
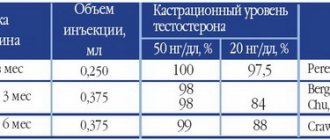
Given the critical importance of testosterone for prostate cancer growth, minimizing the production of this androgen is a prerequisite for successful treatment. Standard methods of androgen deprivation therapy, in particular orchiectomy, and the use of GnRH analogues, lead to the cessation of androgen production in the testes, without having an effect on the production of androgens in the adrenal glands.
Currently, multicenter randomized phase II–III studies are being conducted using new chemotherapeutic agents (cabazitaxel), immunodrugs (ipilimumab, EMD525797, etc.), vaccines (sipuleucel-T), targeted agents, as well as a group of androgen synthesis blockers (abiraterone acetate, orteronel).
The C0U-AA-301 study, which assessed the effectiveness of abiraterone acetate therapy in patients with metastatic castration-resistant PCa (mCRPC) after failure of taxane chemotherapy, included 1195 patients from 13 countries. The first group of patients received abiraterone acetate once daily along with prednisone, and the second group received placebo with oral prednisone. Therapy was continued until progression. The primary objective of the study was to evaluate overall survival (OS), and time to progression (TTP), progression-free survival (PFS), and overall clinical response were also assessed. The median follow-up of patients was 12.8 months. According to study results published by Johann S. de Bono, OS was longer in the abiraterone acetate group than in the placebo plus prednisone group, values of 14.8 months and 10.9 months, respectively. All additional endpoints assessed, such as time to PSA progression (10.2 months and 6.6 months), PFS (5.6 months and 3.6 months) and PSA response rate (29% and 6%), indicated in favor of abiraterone acetate. The study also demonstrated benefits for patients receiving abiraterone acetate therapy compared with a group of patients receiving placebo therapy in the following indicators: quality of life (general functional status), reduction in the degree and duration of pain, reduction in the risk of developing skeletal complications.
Since its first approval in the United States in 2011, Zytiga® has been approved in more than 50 countries. The drug is being administered to more than 30,000 men worldwide and is now rapidly becoming the mainstay treatment option for mCRPC.
The Federal Service for Surveillance in Healthcare and Social Development registered the drug Zitiga® (abiraterone acetate) in Russia in 2012.
From November 2013 to October 2014, 42 patients with hormone-resistant metastatic prostate cancer who had previously received chemotherapy were under our supervision in urological oncology hospitals and clinics of the Moscow City Health Department from November 2013 to October 2014.
Before inclusion in the study, 32 patients received 3-24 courses of Taxotere in the 1st line at a dose of 85 mg/m2 - 1 day, interval 21 days against the background of standard premedication with dexamethasone, of which:
8 people received 3 courses, in 1 patient treatment was canceled due to manifestations of hematological toxicity (persistent neutropenia grade III); 7 of whom showed progression of the disease in the form of the appearance of new metastatic foci in the bones, lungs, lymph nodes (4 patients in the second line of chemotherapy were treated with Cabazitaxel at a dose of 25 mg/m2 1 day in combination with prednisolone 10 mg per day daily , 2 patients received 6 courses of chemotherapy, treatment was completed due to progression of the disease in the form of the appearance of new metastatic foci in the bones, in 1 patient treatment was completed after the 4th course of chemotherapy due to the development of persistent hematological toxicity (anemia II-III), in 1 patient treatment was completed due to symptoms of grade II neuropathy);
10 patients received 6 courses of chemotherapy, in 4 patients progression of the disease was noted in the form of bone metastases, metastases in the lungs and pleura, persistent increase in PSA, in 2 patients hematological toxicity was noted (neutropenia grade III–IV, anemia grade III), in 2 patients - patients with grade II–III asthenia, 2 patients with grade II–III gastrointestinal toxicity;
9 patients received 9 courses of chemotherapy, of which 4 had the appearance of new bone metastases, as well as metastases to the lungs against the background of a persistent increase in PSA, 3 patients had a persistent increase in PSA, 1 patient had hematological toxicity (persistent neutropenia III - stage), 1 patient had grade II–III neuropathy;
4 patients received 12 courses of chemotherapy, in 3 patients progression of the disease was noted in the form of the appearance of new bone metastases, an increase in the number and size of metastases in the lungs and retroperitoneal lymph nodes, and in one patient there was a persistent increase in PSA.
1 patient received 24 courses of chemotherapy; treatment was discontinued due to progression of the disease with the emergence of new bone metastases and a persistent increase in PSA.
10 patients received from 3 to 9 courses of chemotherapy with Mitoxantrone at a dose of 10 mg/2 -1 day with an interval of 21 days and 10 mg of prednisolone per day daily. 1 patient received second-line Cabazitaxel at a dose of 25 mg/m2 for 1 day in combination with prednisolone 10 mg per day daily. The patient underwent 8 courses of Cabazitaxel chemotherapy, treatment was completed due to progression of the disease in the form of the appearance of new bone metastases, as well as metastatic lesions of the retroperitoneal lymph nodes. All patients showed progression of the disease after treatment. Among them, new bone metastases were identified in 7, metastases in the iliac and retroperitoneal lymph nodes were detected and morphologically verified in 6, and metastases in the lungs were detected in 2 patients.
All patients underwent general clinical laboratory tests: complete blood count, biochemical blood test, determination of the level of prostate-specific antigen (PSA) and testosterone, ECG and Echo-CG taking into account the ejection fraction value of at least 50%, plain radiography of the chest, echographic examination of the organs abdominal cavity, transrectal echographic examination of the prostate gland, bladder.
The next stage of the examination was osteoscintigraphy and computed tomography with examination of the abdominal organs, pelvis, regional and distant lymph nodes. In all patients, the quality of life was determined by assessing the activity status according to the Karnofsky system and the level of pain using a four-point system approved by WHO.
Activity status of patients before treatment.
| Gradation | Activity according to Karnovsky, % |
| 80–100 | 21(50%) |
| 60–70 | 11(26,1%) |
| 50–60 | 8 (19,04%) |
| 30–50 | 2(4,7) |
Thus, 21 patients were included with an activity status of 80–100%, 11 with an activity status of 60–70%, and 8 patients with an activity status of 50–60%, 2 patients with an activity status of 30–50 according to the Karnofsky system.
At the start of treatment, all patients had morphological confirmation of the diagnosis of prostate cancer. In most cases these were poorly differentiated forms of cancer. In 100% of cases, we encountered various variants of adenocarcinoma. The distribution on the Gleason scale was as follows: 4 points in 1 patient, 5 points in 6 patients, 6 points in 8 patients, 7 points in 11 patients, 8 points in 10 patients, 9 points in 6 patients.
The age of the patients was distributed between 60 and 84 years. The average age of the patients was 72.2 + 11.8 years. 78.4% of patients were aged 60 years or older. A comprehensive examination before the start of treatment made it possible to identify concomitant diseases of varying severity in 33 patients (78.5%). In 26 patients, the leading place was occupied by diseases of the cardiovascular system: hypertension of varying degrees (including with a history of stroke), chronic ischemic heart disease (including with a history of myocardial infarction), general atherosclerosis, atherosclerosis of the coronary arteries and cerebral vessels, diseases of the gastrointestinal tract - chronic gastritis, chronic cholecystopancreatitis, chronic colitis, obesity.
Abiraterone NV, 250 mg, tablets, 120 pcs.
Taking Abiraterone NV with food significantly increases the absorption of abiraterone acetate. The effectiveness and safety of Abiraterone NV taken with food has not been established. Abiraterone NV should not be taken with food.
Increased blood pressure, hypokalemia and fluid retention due to excess mineralocorticoids
Abiraterone NV may cause increased blood pressure, hypokalemia and fluid retention due to increased mineralocorticoid concentrations due to inhibition of the CYP17 enzyme. Taking corticosteroids reduces the stimulating effect of adrenocorticotropic hormone (ACTH), resulting in a reduction in the frequency and severity of these adverse reactions. Caution should be exercised when treating patients whose clinical condition may be worsened by increased blood pressure, hypokalemia, or fluid retention (eg, patients with heart failure, recent myocardial infarction, or ventricular arrhythmia).
Abiraterone NV should be administered with caution to patients with a history of cardiovascular disease. Safety of the drug in patients with left ventricular ejection fraction
Blood pressure, plasma potassium concentration and degree of fluid retention should be monitored at least once a month.
Hepatotoxicity
In clinical studies, a pronounced increase in the activity of liver enzymes was recorded, which required discontinuation or dose adjustment of the drug. Serum transaminase and bilirubin activities should be measured before starting Abiraterone, every 2 weeks for the first 3 months of treatment, and then monthly. If clinical symptoms and signs suggestive of liver dysfunction develop, serum transaminase activity, in particular alanine aminotransferase, should be immediately measured. If alanine aminotransferase activity increases to 5 times the upper limit of normal or bilirubin concentrations exceeds 3 times the upper limit of normal, Abiraterone NV should be discontinued immediately and liver function should be carefully monitored.
Abiraterone NV can be used again only after liver function tests have returned to baseline values and only when treated with lower doses.
If patients develop severe hepatotoxicity during any period of therapy (alanine aminotransferase activity exceeds the upper limit of normal by 20 times), Abiraterone NV should be discontinued; re-prescribing the drug in such patients is impossible.
No dose adjustment is required in patients with mild hepatic impairment. There are no data on the effectiveness and safety of repeated use of abiraterone acetate in patients with moderate to severe hepatic impairment (Child-Pugh class B or C), so the need for dose adjustment cannot be predicted. Abiraterone NV should be used with caution in patients with moderate hepatic impairment, only if the benefit of treatment clearly outweighs the possible risk. Abiraterone NV should not be prescribed to patients with severe liver dysfunction.
Women of childbearing age
Abiraterone NV is not intended for use in women. Taking CYP17 inhibitors by pregnant women is expected to alter hormone concentrations, which may affect fetal development. To prevent accidental exposure, women who are pregnant or may become pregnant should not handle the product without gloves.
Contraception in men and women
It is unknown whether abiraterone acetate or its metabolites are present in semen. It is necessary to use a condom if you plan to have sexual intercourse with a pregnant woman. If sexual intercourse is planned with a woman of childbearing age, it is necessary to use a condom along with other effective methods of contraception.
Fertility
Studies on the toxicity of abiraterone acetate for the reproductive system have not been conducted; there is no data on the effect of the drug on the ability to conceive.
Cancellation of glucocorticosteroids and relief of stressful situations
When discontinuing prednisolone, caution should be exercised and monitored for signs of adrenal insufficiency. If use of Abiraterone NV is continued after discontinuation of glucocorticosteroids, the appearance of symptoms of mineralocorticoid excess should be monitored. In patients receiving prednisolone, the development of stressful situations may require an increased dose of glucocorticosteroids before, during and after a stressful situation.
Simultaneous administration of Abiraterone NV and chemotherapy
The safety and effectiveness of simultaneous administration of Abiraterone NV and cytotoxic chemotherapy have not been established.
Information about some excipients included in the drug Abiraterone NV
This medicinal product contains 1 mmol (27.2 mg) sodium per dose (4 tablets), which should be taken into account when treating patients on a sodium controlled diet.
Impact on the ability to drive vehicles and operate machinery
The drug Abiraterone NV does not affect or has a negligible effect on the ability to drive a car and move machinery.
Comparative characteristics of patients depending on PSA level before treatment.
| PSA < 10 ng/ml | 0(0%) |
| PSA 10–50 ng/ml | 16(38,1%) |
| PSA > 50 ng/ml | 26 (61,9%) |
Thus, the majority of patients had a prostate-specific antigen level greater than 50 ng/ml (with a normal level of up to 4 ng/ml).
The daily dose of Zytiga for all patients was 1 g (4 tablets of 250 mg) 1 time/day 1 hour before meals or 2 hours after meals.
Zytiga was used together with low doses of prednisolone. The recommended dose of prednisolone was 10 mg/day daily.
Patients with bone metastases received bisphosphonates. Patients received Zoledronic acid at a dose of 4 mg intravenously infusions with an interval of 28 days.
To date, patients have received from 1 to 11.5 months of abiraterone therapy. Upon reaching 6 months of treatment, the dynamics of the therapy was assessed. In case of positive dynamics and functional safety of the patient, treatment continued as before. The minimum treatment time is two months, the maximum is 11.5 months.
At the moment, 2 patients received 2 courses, 6 patients received 3 courses, 2 patients received 4 courses, 4 patients received 5 courses, 8 patients received 6 courses, 9 patients received 7 courses, 4 patients received 8 courses, 3 patients received 9 courses, 2 patients received 10 courses, 1 patient 11 courses and 1 patient 11.5 courses. 28 people (66.6%) received six courses or more. The average treatment time was 8.25 months.
Number of chemotherapy courses performed
| Up to 6 months | ≥ 6 months | ≥11 months | Wed. number of courses | |
| Abiraterone | 14(33,3%) | 26(61,9%) | 2 (4,7%) | 7,42±1,01 |
The traditional fundamental criterion for response in the treatment of prostate cancer is the dynamics of blood PSA levels. In response to treatment, a decrease in PSA levels by more than 50% from the initial level before treatment was observed in 22 patients (52.3%). Of this number, a decrease in PSA levels below 80% was recorded in 7 patients (16.6%). Stabilization of PSA levels was observed in 9 patients (21.4%). Progressive growth of PSA was determined in 11 patients (26.1%).
The leading criterion for the effectiveness of drug chemotherapy is the assessment of the dynamics of PSA levels. Depending on the duration of the chemotherapy cycle, PSA levels were assessed every three weeks (before the planned administration). We assessed the positive dynamics of changes in PSA levels in response to treatment as:
- decrease in PSA level by 50% from baseline before the start of therapy;
- a decrease in PSA levels by 80% or more from baseline to the start of therapy;
- increase in PSA level, i.e. progression;
- stabilization of PSA levels, which was assessed as the absence of progression on the one hand and the absence of a decrease in PSA levels by 50% or more on the other hand.
The period of response based on PSA level was determined from the moment of its decrease until the return to the initial level and above.
To study the objective response from bone metastases, the assessment was carried out using the MS Soloway system. The assessment criteria based on osteoscintigraphy data were:
- absence of new lesions;
- disappearance of previously identified lesions;
- reduction in the % accumulation of radiopharmaceuticals in areas of bone tissue damage.
For patients with lymph node involvement and visceral metastases, the Regression Evaluation Criteria for Measured Solid Tumors (RECIST) were used.
Evaluation of the effect showed the following results: a decrease in PSA levels by more than 50% was recorded in 22 patients, and a decrease in PSA by more than 80% in 7 patients.
Material and methods
The study was conducted from 2013 to early 2015. It included 53 patients with metastatic CRPC (mCRPC) who had previously received chemotherapy (CT) and LHRH antagonists.
In first-line chemotherapy, 35 patients with mCRPC received chemotherapy with docetaxel (5-21 courses) at a dose of 85 mg/m2 with standard premedication with dexamethasone. In 8 patients, persistent hematological toxicity (grade III-IV) was noted, and therefore treatment was discontinued. In the second line of chemotherapy, 13 patients received 5 courses of cabazitaxel 25 mg/m2 in combination with prednisolone 10 mg per day. Severe hematological toxicity (anemia, grade III neutropenia) was observed in 2 patients.
In the first line of therapy, 18 patients with mCRPC, after disease progression during treatment with LHRH agonists and the development of castration resistance, received the LHRH antagonist degarelix subcutaneously at an initial dose of 240 mg and a maintenance dose of 80 mg. In 2 patients, treatment was discontinued due to intolerance to the drug (anemia, diarrhea, nausea, hot flashes) (Table 1).
Table 1. Previous therapy
All patients included in the study underwent regular laboratory tests: general and biochemical blood tests, determination of PSA levels, testosterone, ECG, chest x-ray, transrectal ultrasound (ultrasound) of the prostate gland, ultrasound of the abdominal cavity and pelvic organs, as well as bone scintigraphy and computed tomography of the chest, abdomen, pelvis, and regional and distant lymph nodes.
The quality of life of patients was assessed based on the EORTC questionnaire, activity status according to the Karnofsky system and the level of pain on a five-point scale.
The study included 24 patients with a Karnofsky activity status of 80–100%, 15 patients with a status of 60–70%, 10 patients with a status of 50–60%, and 9 patients with a status of 30–50%.
The median age of the patients was 72.0±7.6 years. Distribution of patients depending on age: there were 5 (9.4%) patients under 60 years old, 30 (56.6%) patients were 60-69 years old, 18 (33.9%) patients were over 69 years old.
At the start of the study, 47 (88.6%) patients complained of urinary disorders, 38 (71.6%) of weakness, 14 (26.4%) of pain of various locations (Table 2).
Table 2. Complaints of patients at the start of the study
For the purpose of histological verification of the diagnosis, a transrectal biopsy of the prostate gland was performed before treatment, followed by assessment of tumor differentiation according to the Gleason score. All patients who underwent a biopsy were prescribed broad-spectrum antibiotics 1 day before the procedure to prevent infectious complications. According to the results of the biopsy, adenocarcinoma was detected in all (100%) patients and the degree of differentiation was determined according to the Gleason score: less than 7 - 7 (13.2%), 7 - 28 (52.8%), more than 7 - 18 (34%).
The average PSA concentration before the start of the study was 134 (6.7–497) ng/ml (Table 3).
Table 3. Distribution of patients depending on PSA level before the start of the study
The prevalence of the tumor process was assessed based on the TNM classification. Of the 53 patients included in the study, stage T2 was in 6 (11.3%) patients, T3 in 29 (54.7%), T4 in 18 (33.9%) (Table 4).
Table 4. Distribution of patients depending on T category according to the TNM classification
Distant metastases (M1) were detected in all patients included in the study (100%). Metastases in the lymph nodes (N1) were found in 36 (67.9%) patients, in the bones - in all patients: 23 (43.3%) of them had less than five metastatic foci, 30 (56.7%) - more than five 27 (50.9%) patients had lesions and metastases in the lungs (Table 5).
Table 5. Distribution of patients depending on the location of metastases
In all 53 patients, the drug abiraterone acetate (Zitiga) was used as the next line of therapy. Abiraterone was used at a daily dose of 1000 mg (4 tablets of 250 mg) 1 hour before a meal or 2 hours after a meal together with prednisolone at a dose of 10 mg per day.
In connection with bone metastases, patients received bisphosphonates (zoledronic acid at a dose of 4 mg intravenously every 28 days) or denosumab at a dose of 170 ml subcutaneously every 4 weeks.
Every 6 months of therapy, the dynamics of treatment and the functional status of patients were assessed. In case of positive dynamics and absence of functional deterioration, therapy continued as before. The minimum course of therapy was 3 months, the maximum was 1 year, the average treatment time was 7.26 courses (Table 6). It should be noted that our results are comparable with literature data on abiraterone therapy in the second line of mCRPC therapy [12]. However, it can be expected that the duration of therapy would average more than 15 months if abiraterone treatment were initiated in the first line of treatment for mCRPC [9].
Table 6. Duration of abiraterone therapy
PSA levels were determined every 3 weeks. Positive dynamics of PSA levels were assessed as a decrease in PSA levels by 50% or more from baseline before the start of abiraterone therapy; stabilization of PSA levels was assessed as the absence of progression and a decrease in PSA levels by 50% or more. The period of response to treatment was determined from the moment the PSA level decreased until it returned to the initial level or higher.
Results of PSA changes in the Abiraterone monotherapy group
| PSA dynamics | |
| PSA decline>80% (%) | 7(16,6%) |
| PSA decline >50% (%) | 22(52,3%) |
| PSA stabilization | 9(21,4%) |
| PSA progression | 11(26,1%) |
Thus, an overall response by PSA level was observed in 31 patients (73.8%). It was noted that in 11 patients with positive PSA dynamics, stabilization of pathological processes in the skeletal system occurred, in 6 patients stabilization was noted in the lymphatic system, partial regression of the lymph nodes was noted in 4 patients; in 6 patients with metastatic lesions of the lungs, including 1 patient with lesions of the pleura, stabilization of the process was noted; in 2 patients, a partial effect was observed in the form of a decrease in the number and size of metastatic foci.
In 11 patients, a progressive increase in PSA levels was noted, of which 5 patients had progression from bone metastases, 2 patients had progression of the disease from the lymphatic system, 3 patients had new lesions in the lungs, enlarged and appeared, 1 patient had a persistent increase The dog had no signs of visceral and bone metastases.
Therefore, from the point of view of changes in PSA, it can be argued that the effectiveness of treatment is statistically significant.
The response to treatment of patients with deposits in the skeletal system in the form of stabilization of the process was observed in 11 cases out of 16 (68.7%). There was stabilization of pathological processes in the bone metastatic focus according to osteoscintiography. The absence of new foci of radiopharmaceutical accumulation at the end of treatment was recorded.
Due to the progression of the tumor process in the bones after 3 courses of chemotherapy, 5 patients were transferred to other treatment options, and a persistent increase in PSA was recorded in these patients.
The defining criterion for the effectiveness of chemotherapy is the life expectancy of patients after completion of treatment, which was 11.5 months.
We analyzed one-year survival rates. One-year survival rate was 71.4%.
Attention should be paid to the fact that 4 patients showed a partial effect on Abiraterone therapy, and all these patients received Taxotere in the 1st line of therapy and were withdrawn from treatment due to the development of various types of toxicity.
In 23 patients, stabilization of the process was noted, of which 8 patients received Taxotere in the 1st line of therapy, treatment was stopped due to the development of various types of toxicity, 13 patients who received Taxotere in the 1st line were transferred to Abiraterone due to development of disease progression. And in 2 more patients, stabilization of the process was noted after Cabazitaxel in the 2nd line of chemotherapy.
Thus, the response to Abiraterone treatment was higher in those patients who were withdrawn from Taxane treatment due to toxicity, and not due to disease progression.
Among grade I–II adverse events, we most often (≥10%) noted peripheral edema, hypokalemia, increased blood pressure, urinary tract infections, and increased ALT or AST activity.
Adverse reactions are systematized for each organ system using the following frequency classification: very common (≥1/10), common (≥1/100, <1/10), uncommon (≥1/1000, <1/100), rare (≥1/10,000,<1/1000), very rare (<1/10,000, including isolated cases).
There were no deaths due to disease progression within 30 days of taking the last dose of the study drug.
Abiraterone dose reduction was not carried out. In case of toxic effects (hepatic and cardiotoxicity) in 4 patients, the drug was discontinued.
In 3 patients, stage III–IV liver toxicity was noted, in 2 patients after the 3rd course and in 1 patient after the 5th course, in the form of an increase in the level of bilirubin, as well as liver transaminases, which was not relieved by hepatoprotective therapy for more than 23 days. In 6 patients, stage I–II liver toxicity was observed, and the use of hepatoprotectors was successfully relieved.
In 1 patient, after 3 courses of treatment, left ventricular failure was noted, a decrease in left ventricular ejection fraction to 38%, and angina pectoris 2 f.k. Treatment was suspended, the patient was given cardioprotective therapy for 46 days, after which a follow-up examination revealed progression of the disease in the form of an increase in PSA, and progression of metastases in the bones was also noted in this patient.
The most common toxic complication was grade 1–2 peripheral edema in 19 patients (45.2%). The swelling was transient during treatment; after stopping treatment in 2 patients due to the development of liver toxicity and in 1 patient due to cardiotoxicity, as well as in 8 patients who dropped out due to disease progression, the edema self-limited without the use of medication. treatment, in 3 patients who dropped out due to disease progression, edema persisted for more than 25 days; these patients received diuretic therapy, with full effect. There was no objective deterioration in the patients' condition. The second most common side effect was hypokalemia, which occurred in 18 patients (42.8%), in 7 cases accompanied by tachycardia, 11 patients suffered asymptomatically. Accompanying therapy was carried out with infusion of Potassium chloride 0.4% 20 ml intravenously until the parameters normalized, followed by permanent oral administration of Panangin.
Arterial hypertension was of a similar moderate nature, observed in 16 patients, which was successfully controlled by taking the ACE inhibitor enalapril 2.5 mg x 2 times a day orally permanently for a long time.
Nausea was observed in 12 patients; it was successfully relieved by taking metoclopramide 10 mg x 4 times a day orally for a long time in 8 patients; 4 patients received antiemetic therapy with ondansetron 16 mg per day parenterally, until symptoms were relieved with a further maintenance regimen of 8 mg per day orally.
Urinary tract infections were noted in 14 patients, treatment was carried out with nitroxoline 600 mg x 3 times a day orally, in 9 cases, on days 7–10 of treatment, complete recovery occurred, in 5 patients it did not bring the desired effect, therapy was carried out with urophosphabol in combination with uroseptics, however In 3 patients, persistent bacteriuria persisted, and therefore treatment with Abiraterone was stopped; in 2 patients, during high-dose antibiotic therapy, a persistent increase in creatinine was noted, and therefore treatment with Abiraterone was also stopped.
Angina pectoris was noted in 6 patients, in 5 cases it was successfully stopped by oral administration of nitroglycerin, in one case the administration of nitroglycerin was parenteral.
In general, there were expected side effects of this chemotherapy regimen, controlled by etiotropic and symptomatic therapy.
results
The criterion for response to treatment was the dynamics of blood PSA levels. In our study, a decrease in PSA levels by more than 50% from baseline was observed in 27 (50.9%) patients. In this group, a decrease in PSA levels below 80% was observed in 15 (28.3%) patients. Stabilization of PSA was observed in 14 (26.4%) patients. A progressive increase in PSA was noted in 12 (22.7%) patients. An overall response based on PSA levels was observed in 40 (75.4%) patients.
Thus, we can assume that changes in PSA levels during abiraterone treatment reflect the clinical effectiveness of therapy.
Due to the progression of the tumor process and a persistent increase in PSA levels after 4 courses of therapy in 7 patients, treatment had to be stopped and they were transferred to other types of therapy.
The objective response of bone metastatic lesions to therapy was assessed using the Soloway system. The evaluation criteria were: absence of new bone lesions, disappearance of previously identified lesions, percentage reduction in the accumulation of radiopharmaceuticals in the affected bones.
For patients with lymph node and lung metastases, response to treatment was determined by Response Evaluation Criteria in Solid Tumors (RECIST 1.1).
When analyzing the study, it was noted that a partial response to therapy was given by both patients who received degarelix and patients who received one or more lines of chemotherapy with taxanes. Responses were also observed among patients in whom taxane chemotherapy was discontinued due to intolerance.
The spectrum of adverse events associated with abiraterone therapy was analyzed. The most common symptoms (more than 10%) were peripheral edema, increased blood pressure, urinary tract infections, and increased liver enzymes (grades I-II). However, most adverse events were easily managed, and the therapy corresponded to a favorable safety profile.
There were no deaths due to disease progression within 30 days of the last dose of abiraterone. During the study, the drug dose was not adjusted; in 6 cases the drug was discontinued due to intolerance (grade III liver and cardiotoxicity). In patients in whom the drug was discontinued, a follow-up examination for concomitant pathology showed progression of bone lesions and a persistent increase in PSA levels.
Other adverse events, such as nausea, vomiting, weakness and pain, were successfully treated with metoclopramide, ondasetron and analgesics. Patients with urinary tract infections were successfully treated with fluoroquinolone antibiotics. It can be concluded that adverse events during abiraterone therapy were consistent with the favorable safety profile of the drug.
The dynamics of the pain status of patients were assessed on a 5-point scale before and after treatment with abiraterone (Table 7).
Table 7. Dynamics of pain status of patients
During treatment, it was noted that 12 patients stopped taking analgesics during the therapy, 3 patients stopped taking narcotic analgesics, and 2 began to take narcotic drugs less often.
Positive dynamics were noted in changes in the activity status of patients (Table 8). After the analysis, it was noted that in 14 patients there was a change in activity status for the better.
Table 8. Dynamics of patient activity status
Manifestation of toxicity of Abiroterone monotherapy regimen
| Toxicity | 1 tbsp. | 2 tbsp. | 3 tbsp. | 4 tbsp. |
| Infectious diseases: - urinary tract infections | 6 | 3 | 5 | 0 |
| From the endocrine system: - insufficiency of the kidney function | 0 | 0 | 0 | 0 |
| From laboratory parameters: - hypokalemia, - hypertriglyceridemia, - increased ALT or AST activity. | 11 1 4 | 7 0 2 | 0 0 0 | 0 0 2 |
| From the musculoskeletal system and connective tissue: - fractures | 0 | 0 | 0 | 0 |
| From the kidneys and urinary tract: - hematuria | 0 | 0 | 0 | 0 |
| From the cardiovascular system: - increased blood pressure; - left ventricular failure, - decreased fraction - of the left ventricle, - angina pectoris - arrhythmia - atrial fibrillation - tachycardia | 12 0 0 4 0 0 7 | 4 0 0 1 0 0 0 | 0 0 1 1 0 0 0 | 0 1 0 1 0 0 0 |
| From the digestive system: - dyspepsia | 2 | 0 | 0 | 0 |
| General disorders: - peripheral edema | 7 | 9 | 3 | 0 |
The quality of life of patients with Abiraterone monotherapy is presented below:
Abiraterone
| Gradation of pain | Number of patients | |
| Before treatment | After treatment | |
| 0 - no analgesics required | 11(26,1%) | 24(57,1%) |
| 1 - sometimes non-narcotic | 14(33,3%) | 10(23,8%) |
| 2 - regularly non-narcotic | 12(28,5%) | 7(16,6%) |
| 3 - sometimes narcotic | 3(7,1%) | 1(2,4%) |
| 4 - regularly use drugs | 2(4,7) | 0 |
As can be seen from the data obtained, the number of patients who do not require taking analgesic drugs increased by 13 people (30.9%) against the background of the therapy. At the same time, we were able to achieve a complete refusal of regular use of narcotic drugs in 1 patient, 1 patient who had previously regularly received narcotic analgesia was not able to completely stop taking narcotic drugs, but reduced the intake to 1 time per day (previously received 2 times a day), 3 patients who had previously received irregular narcotic analgesia were able to completely abandon it in favor of non-narcotic analgesia.
Positive dynamics in this group were also observed in changes in the activity status of patients.
Abiraterone
Taking abiraterone with food significantly increases the absorption of abiraterone. The effectiveness and safety of abiraterone taken with food has not been established. Abiraterone should not be taken with food.
Increased blood pressure, hypokalemia, fluid retention and heart failure due to excess mineralocorticoids
Abiraterone may cause increased blood pressure, hypokalemia, and fluid retention due to increased mineralocorticoid concentrations due to inhibition of the CYP17 enzyme. Taking corticosteroids reduces the stimulating effect of adrenocorticotropic hormone (ACTH), resulting in a reduction in the frequency and severity of these adverse reactions. Caution should be exercised when treating patients whose clinical condition may be worsened by increased blood pressure, hypokalemia, or fluid retention (eg, patients with heart failure, recent myocardial infarction, or ventricular arrhythmia).
Abiraterone should be used with caution in patients with a history of cardiovascular disease. The safety of the drug in patients with left ventricular ejection fraction <50% or with heart failure of functional class III-IV according to the NYHA classification has not been established.
Hypokalemia and increased blood pressure should be corrected before initiating abiraterone.
Blood pressure, plasma potassium concentration and the degree of fluid retention should be monitored at least once a month.
Hepatotoxicity and liver dysfunction
In clinical studies, a pronounced increase in the activity of liver enzymes was recorded, which required discontinuation or dose adjustment of the drug. Serum transaminase and bilirubin activities should be measured before initiation of abiraterone, every 2 weeks for the first 3 months of treatment, and monthly thereafter. If clinical symptoms and signs suggestive of liver dysfunction develop, serum transaminase activity should be measured immediately.
If alanine aminotransferase or aspartate aminotransferase increases to 5 times the upper limit of normal or bilirubin concentrations to 3 times the upper limit of normal, abiraterone should be discontinued immediately and liver function should be closely monitored. Abiraterone can be used again only after liver function tests have returned to baseline values, and only if lower doses are prescribed.
If patients develop severe hepatotoxicity during any period of therapy (alanine aminotransferase or aspartate aminotransferase activity exceeds the upper limit of normal by 20 times), abiraterone should be discontinued; re-prescribing the drug in such patients is not possible.
No dose adjustment is required in patients with mild hepatic impairment. There are no data on the effectiveness and safety of repeated use of abiraterone acetate in patients with moderate to severe hepatic impairment (Child-Pugh class B or C), so the need for dose adjustment cannot be predicted. Abiraterone should not be administered to patients with moderate to severe hepatic impairment.
Women of childbearing age
Abiraterone is not intended for use in women. Taking CYP17 inhibitors by pregnant women is expected to alter hormone concentrations, which may affect fetal development. To prevent accidental exposure, women who are pregnant or may become pregnant should not handle the product without gloves.
Contraception in men and women
It is unknown whether abiraterone or its metabolites are present in semen. It is necessary to use a condom if you plan to have sexual intercourse with a pregnant woman. If sexual intercourse is planned with a woman of childbearing age, it is necessary to use a condom along with other effective methods of contraception.
Fertility
There have been no studies of the toxic effects of abiraterone acetate on the reproductive system, and there is no data on the effect of the drug on the ability to conceive.
Cancellation of glucocorticosteroids and relief of stressful situations
When discontinuing prednisolone, caution should be exercised and monitored for signs of adrenal insufficiency. If abiraterone is continued after discontinuation of corticosteroids, monitor for symptoms of mineralocorticoid excess. In patients receiving prednisolone, the development of stressful situations may require an increased dose of glucocorticosteroids before, during and after a stressful situation.
Bone Density
Men with metastatic castration-resistant prostate cancer may experience decreased bone density. With simultaneous use of abiraterone and glucocorticosteroids, this effect may be enhanced.
Previous use of ketoconazole
Patients previously treated with ketoconazole for prostate cancer may be expected to have a lower response rate to abiraterone therapy.
Hyperglycemia
The use of glucocorticosteroids can lead to hyperglycemia, so blood sugar concentrations should be measured frequently in patients with diabetes mellitus.
Concomitant administration of abiraterone and chemotherapy
The safety and effectiveness of concomitant administration of abiraterone and cytotoxic chemotherapy have not been established.
Effect on the musculoskeletal system
Cases of myopathy have been reported with abiraterone use. Rhabdomyolysis with renal failure has occurred in some patients. In most cases, these conditions developed during the first month of treatment, and recovery occurred after abiraterone was discontinued. Caution should be exercised during concomitant use of abiraterone and other drugs that can cause myopathy/rhabdomyolysis.
Dynamics of the activity status of patients in the Abiraterone monotherapy group
| Gradation | Number of patients | |
| Before treatment | After treatment | |
| 80–100 | 21(50%) | 26(61,9%) |
| 60–70 | 11(26,1%) | 8(19,04%) |
| 50–60 | 8(19,04%) | 9(21,4%) |
| 30–50 | 2(4,7%) | 0 |
| < 30 | 0 | 0 |
A positive change in activity status occurred in 6 patients (14.2%).
Thus, the sequential administration of taxanes - docetaxel (Taxotere) in the first line, cabazitaxel in the second line, abiraterone in the 2nd and 3rd lines of therapy - can increase the life expectancy of patients with metastatic prostate cancer and improve their quality of life.
Authors: I. G. Rusakov, T. N. Skvortsova, S. V. Mishugin.