Experience with the use of atosiban for threatened preterm birth
The effectiveness of atosiban, a tocolytic drug developed specifically for use during spontaneous uterine contractions, was assessed in 19 patients with threatened preterm birth. The drug was highly effective (89.5%). No side effects were noted in any case.
Introduction
Currently, the frequency of preterm births in the world is not decreasing and amounts to 5–10% of all births [1, 2]. Premature children account for 60–70% of cases of early neonatal mortality, 50% of neurological diseases, including cerebral palsy; premature children have a high prevalence of visual impairment, hearing impairment, and severe chronic lung diseases [3, 4].
If at 28–30 weeks of pregnancy it is possible to accelerate lung maturation in the fetus by using corticosteroids, and prolonging pregnancy at 34–37 weeks does not have a significant effect on perinatal mortality rates, then the least favorable rates of perinatal morbidity and mortality are in children born at term up to 28 weeks of pregnancy. According to the literature, during childbirth at 24 weeks of gestation, death occurs in 80% of cases (for childbirth at more than 30 weeks, this figure is reduced to 10%). 65% of babies born between 22 and 26 weeks die in the delivery room or neonatal intensive care unit. All premature babies who survive to 30 months have some form of disability, and in 50% of cases it is severe [5].
Thus, the frequency of premature births in Moscow in 2012 was 5.8% of the total number of births. Of these, 699 births occurred between 22 and 27 weeks (0.54% of the total). There were 754 newborns born at gestational age 22–27 weeks: 362 (48.1%) stillborn and 392 (51.9%) liveborn. Of all live births, only 239 (31.7%) survived 168 hours, 153 (20.2% of all births) died in the first seven days.
About 70–80% of preterm births occur spontaneously or spontaneously, while the incidence of premature rupture of membranes, according to various authors, ranges from 5 to 40%. The remaining causes are associated with complications that arose during pregnancy on the part of the mother or fetus (induced labor): multiple pregnancy (10–30%), severe forms of gestosis (12%), bleeding during pregnancy due to abruption of a normally located placenta or presentation placenta (6–9%), fetal growth restriction (2–4%) [1, 5].
According to the order of the Ministry of Health dated November 1, 2012 No. 572n “On approval of the Procedure for the provision of medical care in the field of obstetrics and gynecology (except for the use of assisted reproductive technologies)”, in case of threat of premature birth, the use of nifedipine, indomethacin (up to the 32nd week) is recommended pregnancy), beta-agonists (if intolerant to nifedipine), atosiban.
Nifedipine is not licensed in our country as a tocolytic therapy. Previous studies on tocolytics have proven that cyclooxygenase inhibitors are effective drugs for prolonging pregnancy. However, their use causes a number of side effects not only on the part of the mother, but also the fetus: decreased renal function, premature closure of the ductus bollus, increased risk of intraventricular hemorrhage and necrotizing enterocolitis. Foreign researchers recommend prescribing beta mimetics for a short period of time only in order to achieve the effect of corticosteroid drugs or for transporting a child in utero
to an obstetric hospital with a neonatal intensive care unit.
Atosiban is a synthetic competitive inhibitor of vasopressin and oxytocin. By binding to oxytocin receptors, atosiban reduces the frequency of uterine contractions and myometrial tone, which leads to inhibition of uterine contractility. Atosiban also binds to vasopressin receptors, thereby inhibiting the effect of the latter.
Atosiban is the only tocolytic drug developed specifically for use during spontaneous uterine contractions. In many European countries, atosiban is the drug of choice for the treatment of threatened preterm birth.
In Russia, atosiban, the drug Tractocil, was registered in 2012, and in January 2013, its effectiveness in patients with threatened preterm birth was assessed at the group’s hospitals.
Material and methods
In total, in the obstetric hospitals of the group (Perinatal Medical Center and Clinical Hospital "Lapino") the drug atosiban was used in 19 patients.
The indications for the prescription of atosiban were the threat of premature birth at 25–34 weeks of pregnancy: six patients (31.6%) at 25–27 weeks, five patients (26.3%) at 28–31 weeks, eight patients (42. 1%) – 32–34 weeks. The observation group did not include patients with preeclampsia, placenta previa, abruption of a normally located placenta, premature rupture of amniotic fluid, intrauterine growth restriction, or chronic fetal hypoxia.
The age of the subjects varied from 24 to 39 years and averaged 32 ± 4.5 years. No concomitant chronic somatic diseases were identified. Of the gynecological diseases, nonspecific colpitis was diagnosed in four (21.0%) pregnant women, and cervical erosion was diagnosed in three (15.9%) pregnant women.
In 12 (63.2%) patients it was the first pregnancy, in seven (36.8%) it was a second pregnancy (of these, the second pregnancy was in four (21%) women, the third in three (15.7%)). From the multiparous group, four (21%) women had a uterine scar after cesarean section. Pregnancy in two (10.5%) patients occurred as a result of in vitro fertilization. Two (10.5%) women had multiple pregnancies (twins). None of the patients had a history of preterm birth.
The effectiveness of the drug atosiban was assessed on the basis of the clinical picture (complaints, external examination data), as well as the results of ultrasound and cardiotocography studies.
All pregnant women complained of nagging pain in the lower abdomen of varying degrees of intensity, of which 15 patients (78.9%) indicated regular uterine contractions for 15 seconds four to six times within an hour.
During a two-manual examination, six (31.6%) patients were diagnosed with shortening of the cervix to 0.5 cm, the cervical canal was passable freely for two fingers, one of the patients had scanty bleeding.
According to a tocographic study, ten (52.6%) patients had increased uterine tone.
Upon admission, 17 patients underwent ultrasound examination with cervicometry through transvaginal access. In six (31.6%) examined, the length of the cervix was from 28 to 35 mm, in nine (47.4%) the length of the closed part was less than 28 mm, and the internal pharynx was funnel-shaped or expanded by 3 mm or more. In two (10.5%) pregnant women, the amniotic sac prolapsed into the lumen of the cervical canal.
The drug atosiban was administered intravenously to all patients in three successive stages according to the following scheme:
1) bolus administration at an initial dose of 6.75 mg;
2) immediately after bolus administration, infusion of the concentrate at a high dose of 300 mcg/min for three hours;
3) long-term (up to 45 hours) infusion of the concentrate at a low dose of 100 mcg/min.
The total dose of atosiban per course of therapy did not exceed 330 mg. Basically, intravenous administration of atosiban was carried out in the maternity ward with subsequent continuation of the course in the pregnancy pathology department. Four (21.0%) pregnant women received a course of atosiban in the pregnancy pathology department.
Research results
Within two to six hours of atosiban administration, 17 (89.5%) pregnant women noted a decrease in the intensity and frequency of uterine contractions. By the end of the course of treatment with atosiban (after 48 hours), these patients had no complaints of nagging pain in the lower abdomen. At the beginning of therapy and after 48 hours, with an objective external examination and according to cardiotocography, the uterus was in normal tone. To prevent neonatal respiratory distress syndrome, all patients were administered dexamethasone 12 mg intramuscularly twice with an interval of 12 hours. Dynamic assessment of the cervix was carried out with ultrasound cervicometry every two weeks: in 17 pregnant women who responded to treatment, no negative dynamics were detected.
Of the 19 women examined, timely birth occurred in 17 (89.5%), of which 12 (73.7%) had spontaneous birth without complications in the intrapartum and postpartum periods. Caesarean section was performed in five (26.3%) patients. In four women, the indication for surgical delivery was the presence of a scar on the uterus after a cesarean section: three were operated on as planned at 39 weeks of pregnancy, in the fourth, a cesarean section was performed on an emergency basis due to premature rupture of amniotic fluid at gestational age 38 -39 weeks. Another woman in labor underwent abdominal delivery due to the onset of acute fetal hypoxia, the cause of which was the tight entanglement of the umbilical cord around the neck.
The birth weight of children ranged from 2900 to 4350 g with an Apgar score of 8–9 points. The newborns were observed in the children's department and were discharged home on the third to fifth day. In the postpartum period, the patients did not experience any complications.
In two (10.5%) pregnant women, therapy with atosiban was ineffective. One patient was admitted at 25–26 weeks with complaints of regular uterine contractions four to five times within an hour. Vaginal examination diagnosed prolapse of the membranes. The patient received two courses of atosiban: at 25–26 weeks and at 26–27 weeks. Ten days after admission, taking into account the onset of regular labor, an increase in body temperature to 38°C, the appearance of purulent discharge, as well as a leukocyte shift to the left in a clinical blood test, the patient was delivered by emergency cesarean section. The fetal weight at birth was 890 g, the child was observed in the pediatric intensive care unit, he was given artificial ventilation for 20 days, after which he was transferred to the second stage of nursing. As a result of using the drug atosiban, this patient was able to prolong her pregnancy by two weeks. At admission, the estimated fetal weight was 670 g, at delivery – 890 g.
Another patient, a multiparous patient, was admitted at 33 weeks of gestation with complaints of cramping pain in the lower abdomen every 8–10 minutes for 15–20 seconds. On vaginal examination, the cervix is effaced, the opening is 2–3 cm, the amniotic sac is intact. Atosiban was administered over 16 hours. Despite the therapy, regular labor developed, and five hours later a spontaneous birth of a fetus weighing 2250 occurred. The child was observed in the pediatric intensive care unit, he was given artificial ventilation for 12 hours.
No side effects were observed during atosiban therapy in any patient.
conclusions
Premature birth is an important medical, social and economic problem, and therefore an urgent issue is the optimization of therapy aimed at prolonging pregnancy. In 89.5% of observed patients, atosiban proved to be a highly effective drug for the treatment of threatened preterm labor. None of the 19 patients had any side effects.
Currently, the number of cases of preterm birth (PB) in the world has no downward trend and amounts to 5-10% of all births [1, 4]. The relevance of the problem of birth defects is due to the high frequency of early neonatal mortality among premature infants (60-70%), neurological diseases (up to 50%), including cerebral palsy, visual impairment, hearing impairment and other chronic diseases [2, 5].
According to the literature [3], when giving birth at 24 weeks of gestation, 80% of newborns die, while when giving birth after 30 weeks of gestation, 90% of newborns survive. Of children born between the 22nd and 26th weeks of pregnancy, 65% die in the maternity ward or neonatal intensive care unit, and all those who survive to 30 months have some form of disability, and in 50% of cases - heavy.
The least favorable indicators of perinatal morbidity and mortality are found in children born before 28 weeks of gestation, while at 28-30 weeks of gestation it is possible to achieve accelerated lung maturation with the help of corticosteroids. Prolonging pregnancy beyond 34-37 weeks does not have a significant effect on perinatal mortality rates [6].
About 70-80% of premature births occur spontaneously, while the number of cases of premature rupture of membranes, according to various authors [1-3], ranges from 5 to 40%. The remaining causes are associated with complications that arose during pregnancy on the part of the mother or fetus (induced labor): multiple pregnancy in the structure of causes of birth control is 10-30%, severe forms of preeclampsia - 12%, bleeding during pregnancy (abruption of a normally located placenta, previa placenta) - 6-9%, fetal growth restriction - 2-4%.
Premature births in Moscow in 2012 accounted for 5.8% of the total number of births. Among them, 699 (0.54%) births occurred between 22 and 27 weeks. An analysis of the survival of newborns born in 2012 at a gestation period of 22-27 weeks showed that 754 newborns were born at these stages of pregnancy, among them 362 (48.1%) were stillborn, 392 (51.9%) were liveborn. Moreover, of all those born, only 239 (31.7%) live births survived 168 hours, 153 (20.2%) died in the first 7 days.
According to the order of the Ministry of Health of the Russian Federation No. 572n dated November 1, 2012 “On approval of the procedure for providing medical care in the field of obstetrics and gynecology (except for the use of assisted reproductive technologies)”, in case of threat of premature birth, the use of nifedipine, indomethacin (up to 32 weeks of pregnancy), β- adrenergic agonists (if nifedipine is intolerant), atosiban.
Nifedipine is not licensed in our country as a tocolytic therapy. Previous studies on tocolytics have proven that cyclooxygenase inhibitors are effective drugs for prolonging pregnancy, however, their use causes a number of side effects not only on the part of the mother, but also the fetus: decreased fetal renal function, premature closure of the ductus bollus, increased risk of intraventricular hemorrhage and necrotizing enterocolitis. Foreign researchers recommend prescribing β-agonists for a short period of time only in order to achieve the effect of corticosteroid drugs or for transporting a child in utero
to an obstetric hospital with a neonatal intensive care unit.
Atosiban is a synthetic competitive inhibitor of vasopressin and oxytocin that binds to membrane-bound receptors on myometrial cells. This leads to the closure of voltage-gated channels in the myometrial cells, resulting in a decrease in the intracellular calcium content, which normally stimulates contractions.
Atosiban is the only tocolytic drug developed specifically for use during spontaneous uterine contractions. In many European countries, atosiban is the drug of choice for the treatment of pregnant women at risk of preterm labor.
In Russia, Tractocil (atosiban) was registered in 2012 and in January 2013, at the Perinatal Medical Center and the Lapino Clinical Hospital, a group assessed the effectiveness of the above drug in patients with the threat of premature birth.
Material and methods
In the group's obstetric hospitals (Perinatal Medical Center and Lapino Clinical Hospital), atosiban was used in 19 patients.
The indication for use was the threat of premature birth at 25-34 weeks of pregnancy: in 6 (31.6%) patients at 25-27 weeks, in 5 (26.3%) with 28-31 weeks of pregnancy, in 8 ( 42.1%) during pregnancy 32-34 weeks. The observation group did not include patients with preeclampsia, placenta previa, placental abruption, premature rupture of amniotic fluid, intrauterine growth restriction, or chronic fetal hypoxia.
The age of the subjects varied from 24 to 39 years and averaged 32±4.5 years. No concomitant chronic somatic diseases were identified. Of the gynecological diseases, nonspecific colpitis was diagnosed in 4 (21.0%) examined, ectopia of the cervix - in 3 (15.9%).
12 (63.2%) patients had their first pregnancy, 7 (36.8%) had a second pregnancy. Pregnancy in 2 (10.5%) patients occurred as a result of in vitro fertilization. Among 19 patients, 12 (63.2%) patients were expected to give birth for the first time, 4 (21%) had a second birth, and 3 (15.7%) had a third birth. In the multiparous group, 4 (21%) women had a uterine scar after cesarean section. In 2 (10.5%) patients the pregnancy was multiple (twins). None of the patients had a history of preterm birth.
The effectiveness of atosiban was assessed on the basis of clinical data (complaints, external examination data), as well as data from ultrasound and cardiotocographic studies.
All pregnant women complained of nagging pain in the lower abdomen of varying degrees of intensity, of which 15 (78.9%) patients indicated regular uterine contractions of 15 seconds 4-6 times within 1 hour.
During a two-manual examination, 6 (31.6%) patients were diagnosed with shortening of the cervix to 0.5 cm, the cervical canal was freely passable for two fingers, and one of the patients had scanty bleeding.
According to a tocographic study, 10 (52.6%) patients had increased uterine tone.
Upon admission, an ultrasound examination with cervicometry through transvaginal access was performed in 17 of 19 patients. In 6 (31.6%) examined, the length of the cervix was from 28 to 35 mm, in 9 (47.4%) the length of the closed part was less than 28 mm, and the internal pharynx was funnel-shaped or dilated from 3 mm or more. In 2 (10.5%) pregnant women, the fetal bladder prolapsed into the lumen of the cervical canal.
Atosiban was administered intravenously to all patients in 3 consecutive stages according to the following scheme:
1. Bolus administration at an initial dose of 6.75 mg.
2. Immediately after the bolus administration, the concentrate was infused at a high dose - 300 mcg / min - for 3 hours.
3. Next, a long-term (up to 45 hours) infusion of the concentrate was carried out at a low dose of 100 mcg/min.
The total dose per course of therapy did not exceed 330 mg.
Basically, intravenous administration of atosiban was carried out in the maternity ward with subsequent continuation of the course in the pregnancy pathology department. Four (21.0%) pregnant women were treated with atosiban in the pregnancy pathology department.
Results and discussion
During the first 2-6 hours of atosiban administration, 17 (89.5%) of 19 pregnant women noted a decrease in the intensity and frequency of uterine contractions. During an objective examination and according to tocography, the uterus was in normal tone. By the end of the course of treatment with atosiban (after 48 hours), the patients had no complaints of nagging pain in the lower abdomen; upon objective external examination and according to CTG, the uterus was in normal tone. In order to prevent respiratory distress syndrome of newborns, all patients were administered dexamethasone 12 mg intramuscularly twice with an interval of 12 hours. Dynamic assessment of the cervix was carried out using ultrasound cervicometry every 2 weeks: in 17 of 19 observed negative dynamics were not detected.
Among the 19 women examined, timely birth occurred in 17 (89.5%), of which 12 (73.7%) had spontaneous birth, without complications in the intrapartum and postpartum periods. Caesarean section was performed in 5 (26.3%) of 17 patients. In 4 women, the indication for surgical delivery was the presence of a scar on the uterus after a cesarean section: 3 were operated on as planned at 39 weeks of pregnancy, in 1 of the above pregnant women, a cesarean section was performed on an emergency basis due to premature rupture of amniotic fluid at term pregnancy 38-39 weeks. Another woman in labor underwent surgical delivery due to the onset of acute fetal hypoxia, the cause of which was the tight entanglement of the umbilical cord around the neck. The weight of children at birth ranged from 2900 to 4350 g, the Apgar score was 8-9 points. All children were observed in the children's department and were discharged home on the 3-5th day. In the postpartum period, the patients did not experience any complications.
In 2 (10.5%) pregnant women, atosiban therapy was ineffective. One patient was admitted at 25-26 weeks of pregnancy with complaints of regular uterine contractions 4-5 times within 1 hour. Vaginal examination diagnosed prolapse of the membranes. The patient received 2 courses of atosiban: at 25-26 weeks and at 26-27 weeks. 10 days after admission, taking into account the onset of regular labor, an increase in body temperature to 38 °C, the appearance of purulent discharge, as well as a leukocyte shift to the left in the clinical blood test, the patient was delivered by cesarean section on an emergency basis. The fetal weight at birth was 890 g. The child was observed in the pediatric intensive care unit, artificial ventilation was performed for 20 days, after which the child was transferred to the second stage of nursing. As a result of the use of atosiban in this patient, it was possible to prolong pregnancy by 2 weeks. Upon admission, the estimated weight of the fetus is 670 g, at delivery - 890 g.
Another patient, multiparous, was admitted at 33 weeks of pregnancy with complaints of cramping pain in the lower abdomen every 8-10 minutes, 15-20 seconds each. A vaginal examination revealed: the cervix is effaced, the opening is 2-3 cm, the amniotic sac is intact. Atosiban was administered for 16 hours. Despite the therapy, regular labor developed; after 5 hours, spontaneous birth of a fetus weighing 2250 g occurred. The child was observed in the pediatric intensive care unit, and artificial ventilation was performed for 12 hours.
No side effects were observed during atosiban therapy in any patient.
Thus, premature birth constitutes a serious medical, social and economic problem, and therefore an urgent issue is the optimization of therapy aimed at prolonging pregnancy.
conclusions
1. It was found that in 89.5% of observed pregnant women with threatened premature birth, Tractocyl (atosiban) turned out to be a highly effective drug.
2. The results of the study showed that none of the 19 patients had side effects from the use of atosiban.
Atosiban
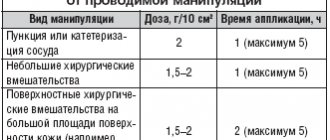
Therapy with Atosiban should be prescribed and administered by a qualified physician experienced in the management of preterm labor. The drug Atosiban is administered intravenously, immediately after diagnosis of premature birth, in 3 consecutive stages: 1) first, 1 bottle of the drug Atosiban, solution for intravenous administration, 6.75 mg/0.9 ml, without dilution, is administered as a bolus over 1 minute (initial dose 6.75 mg); 2) immediately after this, an infusion of the drug Atosiban, concentrate for the preparation of solution for infusion, 37.5 mg/5 ml, at a high dose (loading infusion) of 300 mcg/min is carried out for 3 hours; H) after this, a long-term (up to 45 hours) infusion of the drug Atosiban, concentrate for the preparation of solution for infusion, 37.5 mg/5 ml, at a low dose of 100 mcg/min is carried out. The total duration of treatment should not exceed 48 hours. The maximum dose of Atosiban for the entire course of treatment should not exceed 330.75 mg of Atosiban. Intravenous simultaneous administration of the drug must be carried out immediately after the diagnosis of “premature birth”. After the bolus injection, an intravenous infusion should be started (prepared from a concentrate for solution for infusion containing atosiban 37.5 mg/5 ml). If uterine contractility remains unchanged during therapy with Atosiban, alternative treatment should be considered. The table provides a complete description of the drug dosage regimen for bolus administration and subsequent infusion:
| Stage | Mode | Injection/infusion rate | Atosiban dose |
| 1 | Intravenous bolus injection 0.9 ml over 1 min | Not applicable | 6.75 mg |
| 2 | Intravenous loading infusion over 3 hours | 24 ml/h (300 mcg/min) | 54 mg |
| 3 | Further continuous infusion lasting up to 45 hours | 8 ml/h (100 mcg/min) | up to 270 mg |
Repeated use If it becomes necessary to repeat atosiban, it should also be started with a bolus of 6.75 mg/0.9 ml IV solution (Step 1), followed by an infusion of concentrate to prepare 37.5 mg IV solution. /5 ml (stages 2 and 3). Use of the drug in special clinical groups of patients In postnatal girls under the age of 18 years The effectiveness and safety of atosiban in pregnant women under the age of 18 years have not been established. In patients with renal insufficiency The use of atosiban in patients with renal insufficiency has not been studied. No dose adjustment is required in such patients, since very little atosiban is excreted through the kidneys. In patients with hepatic impairment There are no clinical data on the use of atosiban in patients with hepatic impairment. Atosiban should be used with caution in patients with impaired liver function. Method of use of the drug Stage 2 and 3. Preparation of solution for infusion Before administering the solution, the bottles should be visually inspected for the presence of undissolved particles and changes in the color of the solution. For intravenous infusion, which is carried out immediately after administration of the bolus dose, the concentrate for preparing a solution for infusion, 37.5 mg/5 ml, is diluted in one of the following solutions: - 0.9% sodium chloride solution; - Ringer's solution; - 5% glucose solution. From a bottle containing 100 ml of one of the above solutions for dilution, 10 ml are taken. Then 10 ml (2 bottles of 5 ml) of Atosiban, concentrate for solution for infusion, 37.5 mg/5 ml, is added to the vial to obtain a concentration of 75 mg of atosiban per 100 ml. The solution obtained in this way should be transparent, colorless and free of undissolved particles. A loading infusion is carried out by administering the prepared solution at a rate of 24 ml/h (i.e. 18 mg/h) for 3 hours. The drug should be administered under appropriate medical supervision in the obstetric department. After 3 hours, the infusion rate is reduced to 8 ml/hour. To continue the infusion, it is necessary to prepare the next 100 ml of solution using the method described above. It is necessary to recalculate the amount of the drug to maintain the specified proportion if using a container of a different volume. In order to achieve accurate dosing of the drug, it is necessary to calibrate the rate of administration in the device for dosed intravenous infusion in drops per minute. An intravenous microdrip infusion chamber (infusion pump) can provide a convenient range of infusion rates within the recommended doses of Atosiban. If simultaneous intravenous administration of other drugs is necessary, it is possible to use a divided cannula for intravenous administration or use a different injection site. This method allows for independent control of the infusion rate during administration. The shelf life of the prepared solution for infusion is 24 hours.