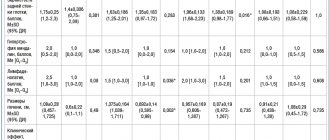
Pharmacological properties of the drug Emla
EMLA cream contains lidocaine and prilocaine, amide-type local anesthetics. Due to the penetration of lidocaine and prilocaine into the layers of the epidermis and dermis, skin anesthesia occurs. The degree of anesthesia depends on the time of application and dose. Intact skin When EMLA cream is applied to intact skin for 1–2 hours, anesthesia lasts about 2 hours after removing the occlusive sticker. There were no differences in efficacy (including time to achieve analgesic effect) and safety when applied to intact skin between young and elderly patients. Due to the effect of the cream on the superficial vessels, temporary pallor or redness of the skin area is possible. Vasomotor reactions develop faster (already 30–60 minutes after applying the cream) in patients with widespread neurodermatitis (atopic neurodermatitis), which indicates a faster penetration of the cream through the changed skin. During a puncture biopsy (up to 4 mm in diameter), the use of the cream provides adequate anesthesia of intact skin in 90% of patients 60 minutes after applying the cream when the needle is inserted into the skin to a depth of 2 mm and after 120 minutes - to a depth of 3 mm. The effectiveness of the cream does not depend on the color and pigmentation of the skin (skin types I–IV). The cream can be used for pain relief before vaccination when administering the vaccine subcutaneously or intramuscularly. Genital mucosa The anesthetic effect of the mucous membrane appears earlier, since absorption occurs faster than in cases of application to intact skin. In women, after applying EMLA cream to the mucous membrane of the genital organs, after 5–10 minutes an analgesic effect is achieved that is sufficient for manipulations using an argon laser. The duration of anesthesia is 15–20 minutes (taking into account individual characteristics - from 5 to 45 minutes). Trophic ulcers of the lower extremities After applying the cream when treating trophic ulcers of the lower extremities, the duration of the analgesic effect is about 4 hours. The cream does not have a negative effect on the wound healing process and bacterial flora. Systemic absorption depends on the amount of cream, duration of application, skin thickness (which varies in different areas of the body) and other skin characteristics. Intact skin In adults, after applying 60 g of EMLA cream to 400 cm2 of intact skin on the thigh (1.5 g per 10 cm2) over 3 hours, systemic absorption was 3% for lidocaine and 5% for prilocaine. Absorption occurs slowly. At the above dose, the maximum plasma concentration of lidocaine (average 0.12 mcg/ml) and prilocaine (average 0.07 mcg/ml) was achieved approximately 4 hours after application. The risk of developing toxic symptoms exists only at doses of 5–10 mcg/ml. Trophic ulcers of the lower extremities After applying 5–10 g of EMLA cream to trophic ulcers with an exposure of 30 minutes, the maximum levels of lidocaine and prilocaine in the blood plasma were achieved after 1–2.5 hours (lidocaine concentration in the range of 0.05–0.84 μg/ml , prilocaine - 0.02–0.08 mcg/ml). After repeated application of EMLA cream to trophic ulcers, no significant accumulation of lidocaine, prilocaine or their metabolites in the blood plasma was noted (EMLA cream was applied at a dose of 2–10 g for 30–60 min to an area of 62 cm2 15 times a month for 3–7 sessions per week). Genital mucosa The maximum plasma concentration is achieved approximately 35 minutes after applying 10 g of EMLA cream to the vaginal mucosa with an exposure of 10 minutes (the average concentration of lidocaine was 0.18 mcg/ml; prilocaine - 0.15 mcg/ml) .
Emla cream for local and external use 30g
Compound
For 1 g of cream, active ingredients: lidocaine 25.0 mg, prilocaine 25.0 mg;
excipients: macrogol glyceryl hydroxystearate (ARLATON 289) 19.0 mg, carbomer 974 P (carboxypolymethylene) 10.0 mg, sodium hydroxide 5.2 mg to bring the pH to 8.7 -9.7, purified water to 1.0 g .
Pharmacokinetics
Systemic absorption of EMLA cream depends on the dose, duration of application and thickness of the skin (depending on the body area), as well as other skin characteristics such as skin diseases and shaving. When applied to the ulcerative surface of the lower extremities, the absorption of the drug may be influenced by the characteristics of the ulcers, for example, the size (with an increase in the area of the ulcer, absorption increases).
Intact skin: In adults, after applying 60 g of cream to an area of 400 cm2 of intact thigh skin (1.5 g per 10 cm2) for 3 hours, systemic absorption for lidocaine was approximately 3% and for prilocaine 5%.
Absorption is slow. The maximum concentration of lidocaine (average value 0.12 μg/ml) and prilocaine (average value 0.07 μg/ml) in blood plasma was reached approximately 4 hours after application of the cream. The risk of toxic symptoms exists only when the concentration of active substances in the blood plasma is 5-10 mcg/ml. When EMLA cream is applied to intact skin 8-12 hours after shaving, the maximum plasma concentration of lidocaine and prilocaine in both young and elderly patients is very low and well below possible toxic levels.
Trophic ulcers of the lower extremities: The time to reach the maximum concentration of lidocaine (0.05-0.84 mcg/ml) and prilocaine (0.02-0.08 mcg/ml) in the blood plasma is 1-2.5 hours from the moment of application of the drug on the ulcer surface (5 - 10 g of cream for 30 minutes). With repeated application of the cream to the ulcerative surface, there was no accumulation of prilocaine, lidocaine or their metabolites in the blood plasma. 2-10 g of EMLA cream were applied to the ulcer surface with an area of up to 62 cm2 for 30-60 minutes from 3 to 7 times a week (15 times within a month).
Genital mucosa: The time to reach the maximum concentration of lidocaine and prilocaine in the blood plasma (on average 0.18 mcg/ml and 0.15 mcg/ml, respectively) is approximately 35 minutes from the moment the drug is applied to the vaginal mucosa (10 g of cream for 10 min).
Indications for use
In adults:
-superficial anesthesia of the skin during punctures (including vaccinations), punctures and catheterization of blood vessels and superficial surgical interventions, including minor cosmetic procedures and hair removal;
-superficial anesthesia of trophic ulcers of the lower extremities during surgical treatment (mechanical cleaning), for example, to remove fibrin, pus and necrotic tissue;
-superficial anesthesia of the mucous membrane of the genital organs before painful manipulations and for pain relief before injections of local anesthetics.
In children:
— superficial anesthesia of the skin during injections (including vaccinations), punctures and catheterization of blood vessels and superficial surgical interventions (including removal of molluscum contagiosum).
Contraindications
- Hypersensitivity to amide-type local anesthetics or any other component of the drug; - premature newborns born at a gestational age of less than 37 weeks; - newborns weighing less than 3 kg.
With caution: Glucose-6-phosphate dehydrogenase deficiency, hereditary or idiopathic methemoglobinemia, common neurodermatitis (atopic dermatitis), patients taking class III antiarrhythmic drugs (for example, amiodarone) (see section “Special Instructions”).
Directions for use and doses
Externally, on the skin or mucous membrane.
Adults. Superficial anesthesia of intact skin
| Indication | Dose and method of application | Application time |
| When inserting a needle, for example when catheterizing blood vessels and taking blood samples | Apply half a 5 g tube (approximately 2 g) per 10 cm2 in a thick layer to the skin and cover with an occlusive dressing | 1 hour, maximum 5 hours |
| For minor surgical procedures, such as curettage of molluscum contagiosum, removal of warts, minor cosmetic procedures and hair removal | 1.5-2 g/10 cm2 apply a thick layer to the skin and cover with an occlusive dressing | 1 hour, maximum 5 hours |
| On large areas of freshly shaved skin (on an outpatient basis), including before epilation | maximum recommended dose 60 g, maximum recommended application area 600 cm2; apply a thick layer to the skin and cover with an occlusive dressing | 1 hour, maximum 5 hours |
| For superficial procedures on large areas (in stationary conditions), for example, taking a rut using the split flap method | 1.5-2 g/10 cm2 apply a thick layer to the skin and cover with an occlusive dressing | 2 hours, maximum 5 hours |
Superficial anesthesia of trophic ulcers of the lower extremities: During surgical treatment (mechanical cleaning) of trophic ulcers of the lower extremities: a single dose of about 1-2 g/10 cm2; Apply the cream in a thick layer to the ulcer surface, no more than 10 g of cream per procedure. Apply an occlusive dressing. Application time: minimum 30 min. An opened tube of cream is intended for single use; the tube with any remaining cream should be discarded after use on one patient. In the case of treating ulcers, into the tissues of which penetration of the drug is difficult, the duration of application can be increased to 60 minutes. Mechanical cleaning must begin no later than 10 minutes after removing the cream.
When manipulating ulcers of the lower extremities, EMLA cream was used up to 15 times over 1-2 months without reducing the effectiveness and increasing the incidence of local reactions. Superficial anesthesia of the genital organs: Skin of the genital organs: Anesthesia before injections of local anesthetics: Men: 1 g/10 cm2. Apply the cream in a thick layer to the skin. Application time: 15 min. Women: 1-2 g/10 cm2. Apply the cream in a thick layer to the skin. Application time: 60 min. Superficial anesthesia of the genital mucosa: When removing condylomas and for pain relief before injections of local anesthetics: approximately 5-10 g of cream, depending on the area of the treated surface. The cream should be applied to the entire surface of the mucous membrane, including the folds of the mucous membrane. No occlusive dressing is required. Application time: 5-10 min. Carry out the procedure immediately after removing the cream.
Children Pain relief during needle insertion (including vaccination), curettage of molluscum contagiosum and other minor superficial surgical procedures. Apply the cream in a thick layer to the skin and cover with an occlusive dressing. The dose should correspond to the surface being treated and should not exceed 1 g of cream per 10 cm.
| Age | Application area | Duration of application |
| 0-3 months | maximum 10 cm2 (total 1 g of cream) (maximum daily dose) | 1 hour (important: no more than 1 hour) |
| 3-12 months | maximum 20 cm2 (total 2 g cream) | 1 hour |
| 1-6 years | maximum 100 cm2 (total 10 g of cream) | 1 hour, maximum 4 hours |
| 6-12 years | maximum 200 cm2 (total 20 g of cream) | 1 hour, maximum 4 hours |
Storage conditions
Store at temperatures below 30°C, out of the reach of children. Do not freeze.
Best before date
3 years. Do not use after the expiration date stated on the package.
special instructions
Patients with glucose-6-phosphate dehydrogenase deficiency or hereditary or idiopathic methemoglobinemia are more susceptible to drug-dependent methemoglobinemia. The effectiveness of using the cream in newborns during the procedure of taking blood samples from the heel has not been established. Caution should be used when applying EMLA cream around the eyes as the drug causes eye irritation.
Elimination of protective reflexes can cause irritation or damage to the cornea. If the cream gets into your eyes, immediately rinse your eyes with water or 0.9% sodium chloride solution and protect your eyes until protective reflexes are restored. Caution must be exercised when applying the drug to the skin with atopic dermatitis; Application time should be reduced (15-30 minutes).
In children under 3 months of age, the safety and effectiveness of EMLA cream was determined after a single dose. In such children, after applying the cream, a temporary increase in the level of methemoglobin in the blood was often observed, lasting up to 13 hours. However, the observed increase in blood methemoglobin levels is probably not clinically significant. Patients taking class III antiarrhythmic drugs (for example, amiodarone) should be under constant monitoring and ECG monitoring, because Possible effect on cardiac activity.
EMLA cream should not be applied to a damaged eardrum or in other cases of possible penetration of the cream into the middle ear.
The cream should not be applied to open wounds. Due to the lack of data on the absorption of the drug, it is not recommended to apply the cream to the genital mucosa in children. Lidocaine and prilocaine in concentrations above 0.5-2% have bactericidal and antiviral properties.
In this regard, it is recommended to take special care when applying the cream before subcutaneous administration of a live vaccine (for example, BCG). Due to the lack of data, the combined use of EMLA cream and drugs that cause methemoglobinemia is not recommended in children aged 0 to 12 months.
Description
Homogeneous white cream.
Conditions for dispensing from pharmacies
Over the counter
Dosage form
Cream for local and external use.
Manufacturer and organization accepting consumer complaints
AstraZeneca AB
Pharmacodynamics
EMLA cream contains lidocaine and prilocaine, which are amide-type local anesthetics, as active ingredients. Skin anesthesia is caused by the penetration of lidocaine and prilocaine into the layers of the epidermis and dermis. The degree of anesthesia depends on the dose of the drug and the duration of application.
Intact skin. After applying EMLA cream to intact skin for 1-2 hours, the duration of anesthesia after removing the occlusive dressing is 2 hours. There were no differences in efficacy (including time to achieve analgesic effect) and safety when applying the cream to intact skin between elderly (65-96 years) and younger patients. Due to the effect of EMLA cream on superficial vessels, temporary paleness or redness of the skin is possible. Similar reactions in patients with widespread neurodermatitis (atopic dermatitis) may occur faster, within 30-60 minutes after applying the cream, which indicates faster penetration of the cream through the skin.
For puncture biopsy (4 mm in diameter), the use of EMLA cream provides adequate anesthesia of intact skin in 90% of patients 60 minutes after application of the cream when the needle is inserted to a depth of 2 mm and after 120 minutes when the needle is inserted to a depth of 3 mm. The effectiveness of EMLA cream does not depend on skin color or pigmentation (skin types I-IV).
When using combined vaccines against infections such as measles, rubella, mumps, or intramuscular combined vaccines against diphtheria, whooping cough, tetanus, polio and infection caused by Haemophilius influenzae type B, as well as vaccination against hepatitis B, the use of EMLA cream had no effect on the average antibody titer, the rate of appearance or disappearance of specific antibodies in the blood serum, or the number of patients who achieved a protective or positive antibody titer after immunization. Genital mucosa Anesthesia of the genital mucosa is achieved more quickly compared to anesthesia of intact skin due to faster absorption of the drug.
In women, 5-10 minutes after applying EMLA cream to the mucous membrane of the genital organs, anesthesia sufficient to relieve pain caused by the use of an argon laser is achieved; The duration of anesthesia is 15-20 minutes (taking into account individual characteristics from 5 to 45 minutes).
Trophic ulcers of the lower extremities. After applying the cream when treating trophic ulcers of the lower extremities, the duration of pain relief is up to 4 hours. There was no negative effect of the drug on the healing process of ulcers or on bacterial flora.
Use during pregnancy and breastfeeding
Pregnancy. There is insufficient data on the use of EMLA cream in pregnant women. Animal studies did not reveal any direct or indirect negative effects of the drug on pregnancy, intrauterine development of the fetus, the process of childbirth or postnatal development. Lidocaine and prilocaine penetrate the placental barrier and can be absorbed into fetal tissues. No specific reproductive effects, such as increased incidence of malformations or other direct or indirect adverse effects on the fetus, have been reported.
Lactation. Lidocaine and prilocaine are excreted in breast milk in quantities that do not pose a risk to the baby when the drug is used in therapeutic doses.
Interaction
In patients receiving drugs that induce the development of methemoglobinemia (for example, drugs containing a sulfo group), EMLA® cream may help increase the concentration of methemoglobin in the blood. When treating with other local anesthetics and structurally similar drugs (including tocainide), the risk of increased systemic effects when using high doses of EMLA cream should be taken into account. No specific studies have been conducted to evaluate the interaction of lidocaine/prilocaine with class III antiarrhythmic drugs; caution should be exercised when using drugs together.
Pharmaceutical interaction: not detected. Drugs that reduce the clearance of lidocaine (eg, cimetidine or beta-blockers) may cause potentially toxic plasma concentrations when repeated high doses of lidocaine are administered over an extended period of time. This interaction is not clinically significant during short-term therapy with lidocaine (eg, EMLA® cream) at recommended doses.
Overdose
If the recommended dosage regimen is followed, the development of signs of systemic toxicity is unlikely. Symptoms of intoxication are likely to be the same as with other local anesthetics, such as central nervous system (CNS) stimulation and, in severe cases, CNS and cardiac depression.
In rare cases, the development of clinically significant methemoglobinemia has been observed. Prilocaine in high doses can cause an increase in methemoglobin levels. Superficial application of prilocaine 125 mg for 5 hours caused the development of moderate methemoglobinemia in a 3-month-old child. Superficial application of lidocaine at a dose of 8.6 - 17.2 mg/kg caused serious intoxication in newborns.
Treatment. Severe neurological symptoms (convulsions, depression of the central nervous system) require symptomatic treatment, including the prescription of anticonvulsants and, if necessary, artificial ventilation. In case of development of methemoglobinemia, the antidote is methylthioninium chloride (methylene blue). Due to the slow systemic absorption of the drug, patients should be monitored for several hours after starting treatment for intoxication.
Impact on the ability to drive vehicles and operate machinery
Does not affect the ability to drive vehicles or operate equipment.
Use of Emla
Adults:
Intact skin | Dose and Application | Duration of application |
| When inserting needles, for example to catheterize blood vessels or take blood samples | Tube volume (about 2 g) per 10 cm2. Apply a thick layer of cream to the skin and cover with an occlusive sticker | 1 hour; maximum 5 hours |
| For minor surgical procedures on the surface of the skin, such as the removal of warts | 1.5–2 g per 10 cm2. Apply a thick layer of cream to the skin and cover with an occlusive sticker | 1 hour; maximum 5 hours |
| When performing surgical procedures on large areas of the skin surface, such as split-flap skin harvesting | 1.5–2 g per 10 cm2. Apply a thick layer of cream to the skin and cover with an occlusive sticker | 2 hours; maximum 5 hours |
Trophic ulcers of the lower extremities During surgical treatment (mechanical cleansing) of trophic ulcers, apply 1–2 g of cream per 10 cm2 of surface. The cream is applied in a thick layer to the surface of the ulcer, no more than 10 g of cream per procedure. Cover the surface of the ulcer with an occlusive sticker. Duration of cream application is 30 minutes. The open tube is intended for one-time use; after each procedure, the unused cream is thrown away. When treating ulcers into which tissue penetration of the drug is difficult, the duration of application can be increased to 60 minutes. Surgical treatment of the wound surface should begin no later than 10 minutes after removing the cream. In the treatment of trophic ulcers of the lower extremities, EMLA cream is used up to 15 times over 1–2 months without reducing effectiveness or increasing the incidence of local reactions. Superficial anesthesia of the genital organs Skin of the genital organs Application of local anesthetics before injection:
- men: 1 g per 10 cm2. A thick layer of cream is applied to the skin. Duration of application - 15 minutes;
- women: 1–2 g per 10 cm2. A thick layer of cream is applied to the skin. Duration of application - 60 minutes.
Mucous membrane of the genital organs To remove condylomas or before injection of local anesthetics: 5–10 g of cream, depending on the area of treatment, is applied to the entire surface, including the folds of the mucous membrane. No occlusal adhesive is required. Application duration is 5–10 minutes. The surgical procedure should begin immediately after removing the cream. Children When performing percutaneous injections, removal of molluscum contagiosum and other superficial surgical manipulations: the dose of EMLA cream should not exceed 1 g per 10 cm2 of surface, the cream is applied in a thick layer to the surface of the skin and covered with an occlusive sticker.
Age | Application area | Duration of application |
| 0–3 months | Maximum 10 cm2 (total 1 g) (maximum daily dose) | 1 hour (note: no longer) |
| 3–12 months | Maximum 20 cm2 (total 2 g) | 1 hour |
| 1 year–6 years | Maximum 100 cm2 (total 10 g) | 1 hour; maximum 5 hours |
| 6–12 years | Maximum 200 cm2 (total 20 g) | 1 hour; maximum 5 hours |
Children with atopic dermatitis need to reduce the duration of application to 30 minutes. Recommendations regarding the method of use Pierce the sealed membrane of the tube with a spike located in the upper outer part of the lid. Squeeze out the required amount of cream and apply to the procedure site. When anesthetizing the skin, occlusive stickers are used, included in the kit with the cream (12 pieces, size 6x7 cm).
Where is topical anesthesia used?
Most often it is used for pain relief when puncturing the skin during catheter insertion, injections, blood sampling, as well as in the following areas of medicine, such as:
- dentistry (for removing teeth and tartar, to relieve the gag reflex during procedures);
- surgery (for superficial surgical interventions. For example, when harvesting a flap for skin grafting, during mechanical cleaning of trophic ulcers);
- dermatology (for removal of molluscum contagiosum);
- cosmetology (before tattooing, hair removal, mesotherapy, laser therapy);
- gynecology (for removal of condylomas);
- pediatrics (for blood sampling, vaccinations).
Side effects of Emla
True adverse reactions caused by the use of local anesthetics occur with a frequency of 1/1000 patients.
Often (1/100) | Skin: temporary local skin reactions at the application site, such as local blanching, redness, swelling |
Uncommon (1/100–1/1000) | Skin: after application, slight pain, itching (at the application site) |
Rarely (≤1/1000) | General: allergic reactions, in the most severe cases anaphylactic shock. Methemoglobinemia in children |
Isolated cases of local reactions at the site of application of the cream, such as hemorrhagic rashes or petechiae, have been reported, especially after prolonged application in children with atopic dermatitis or warts. If the drug accidentally gets into the eyes, corneal irritation may occur.
Special instructions for the use of Emla
Patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia are more likely to develop drug-dependent methemoglobinemia. EMLA cream should be used with caution on the skin in the eye area, since the drug irritates the mucous membrane of the eyes. In addition, loss of protective reflexes due to anesthetic contact with the cornea can lead to irritation and damage to the cornea. If EMLA cream gets into your eyes, they should be immediately rinsed with water or saline (sodium chloride solution) and protected until sensitivity of the cornea is restored. In patients with atopic dermatitis, the cream should be used with caution, and the duration of application should be reduced to 15–30 minutes. The effectiveness of using the cream in newborns when taking blood from the heel has not been established. The safety and effectiveness of the drug in children under 3 months of age have been studied only using a single dose. In such children, after applying the cream, a temporary increase in the level of methemoglobin in the blood is often observed, lasting up to 13 hours. However, this fact is probably not clinically significant. The cream should not be applied to a damaged eardrum and should not be used in situations where the drug may penetrate into the middle ear cavity. The cream should not be applied to open wounds. The drug should not be applied to the genital mucosa in children due to insufficient data regarding absorption. At concentrations exceeding 0.5–2.0%, lidocaine and prilocaine exhibit bactericidal and antiviral properties. Therefore, if the drug is used during vaccination (intradermal administration of a live vaccine, for example BCG), careful monitoring of the results of vaccination is necessary. EMLA cream should not be used in children aged 0 to 12 months receiving concomitant therapy with drugs that induce methemoglobinemia due to the lack of sufficient clinical experience with its use. It is necessary to monitor patients receiving antiarrhythmic drugs belonging to class III (for example, amiodarone), taking into account the results of the ECG in such patients, since lidocaine and class III antiarrhythmic drugs have additive effects. Pregnancy and lactation period Data regarding treatment of pregnant women with EMLA cream are insufficient. The results of studies conducted on animals do not contain sufficiently complete and reliable information regarding the effect on pregnancy, embryonic/fetal development, childbirth and development after birth. Lidocaine and prilocaine penetrate the placental barrier and can be absorbed by fetal tissues. There were no reports of reproductive disturbances, such as an increased incidence of malformations or direct or indirect effects on the fetus. However, when using the drug during pregnancy, it is necessary to weigh the benefit/risk ratio. Lidocaine and, probably, prilocaine pass into breast milk, but in such small quantities that the effect of therapeutic doses of the drug on the child is unlikely. Does not affect the ability to drive vehicles and operate machinery.
Experience with the use of local anesthetic drug EMLA in children
Pain is a physiological reaction that informs us about harmful effects that damage or pose a potential danger to the body. But pain itself is both a warning signal about trouble in the body and, at the same time, a mechanism that helps develop the ability to adapt to the outside world and protect the body from aggressive environmental factors. Thus, by withdrawing his hand from the flame, the child gains invaluable life experience, which gives him the opportunity to avoid this danger in the future. When performing various injections in children, a negative reaction occurs, depending on age, pain tolerance, injection area, needle diameter and depth of penetration. The vast majority of children experience fear due to anticipation of an injection [1–3,5]. According to psychologists, if a child once experienced pain under certain conditions (visiting a doctor, receiving a vaccination), then if a similar situation arises, he may develop a negative reaction to it, associated with the fear of experiencing pain again. Naturally, if from birth such manipulations as vaccinations are carried out using anesthesia, the child will have no reason to be afraid when visiting the doctor again, meeting with the nurse, etc. [4,6,8]. Infiltration of tissues with anesthetic solutions, such as lidocaine, leads to pain at the time of injection, discomfort and burning sensation caused by the action of the anesthetic [7]. Therefore, the use of the local anesthetic drug EMLA in children is relevant. EMLA is a unique cream for anesthesia of various superficial manipulations on the skin and mucous membranes. EMLA is the English abbreviation EMLA - eutectic mixture of local anaesthetics (eutectic mixture of local anesthetics). EMLA is a mixture of two local anesthetics - lidocaine and prilocaine - in a 1:1 ratio, dissolved in water. The drug is available in the form of 5% cream and patch. One gram of cream or one patch contains 25 mg of lidocaine and 25 mg of prilocaine. EMLA cream was registered in Russia in 1999. It is used to anesthetize any superficial painful manipulations on the skin and mucous membranes. Most of its consumers so far have been in cosmetology. Using EMLA cream, many cosmetic procedures such as hair removal, tattooing, mesotherapy, laser procedures, etc. are used to relieve pain. The cream is used in gynecology and urology, for example, when removing condylomas. There is experience in our country in using the cream in dermatology to remove molluscum contagiosum. You can use the cream when harvesting a skin flap for skin transplantation, for pain relief during surgical cleaning of trophic ulcers. In pediatrics, experience in the use of EMLA cream has been accumulated by pediatric anesthesiologists, hematologists and oncologists - in those areas where children undergo many painful procedures (punctures, catheter placements, blood draws, etc.) [10–13]. The drug is intended for superficial anesthesia of the skin and mucous membranes. The effect of the drug is ensured by its constituent components - lidocaine and prilocaine, which are amide-type local anesthetics. Local anesthetics, penetrating into the layers of the epidermis and dermis, cause anesthesia of the skin [2,9]. The degree of pain relief depends on the dose of the drug and the duration of application. After applying the cream to intact skin for 1–2 hours, the duration of anesthesia after removing the occlusive dressing is 2 hours. For puncture biopsy, the use of EMLA cream provides adequate anesthesia of intact skin in 90% of patients after 60 minutes. after application. Anesthesia of the genital mucosa is achieved faster than that of intact skin due to faster absorption of the drug. In women, after 5–10 minutes. after applying EMLA cream to the mucous membrane of the genital organs, anesthesia is achieved sufficient to relieve pain caused by the use of an argon laser; The duration of action is 15–20 minutes. (taking into account individual characteristics from 5 to 45 minutes). When treating trophic ulcers of the lower extremities, the duration of anesthesia after applying the cream is up to 4 hours. There is no negative effect of the drug on the healing process of ulcers or on bacterial flora. Systemic absorption of the cream depends on the dose, duration of application and thickness of the skin (depending on the area of the body), as well as on other features of the skin. In adults, after applying 60 g of cream to intact skin of the thigh with an area of 400 cm2 (0.2 g per 10 cm2) for 3 hours, systemic absorption of lidocaine is approximately 3%, prilocaine - 5%. Absorbed slowly. Cmax of lidocaine and prilocaine in blood plasma was achieved approximately 4 hours after application of the cream and was 0.12 μg/ml for lidocaine and 0.07 μg/ml for prilocaine. In the treatment of trophic ulcers of the lower extremities, the time to reach Cmax of lidocaine (0.05–0.84 μg/ml) and prilocaine (0.02–0.8 μg/ml) in blood plasma is 1–2.5 hours from the moment of application of the drug on the ulcer surface (5–10 g of cream for 30 minutes). With repeated application of the cream to the ulcerative surface, accumulation of prilocaine, lidocaine and their metabolites is not observed. When applying the cream to the genital mucosa, the time to reach Cmax of lidocaine and prilocaine (0.18 mcg/ml and 1.15 mcg/ml, respectively) is approximately 35 minutes. from the moment of applying the drug to the vaginal mucosa (10 g of cream for 10 minutes). In patients with advanced neurodermatitis, the rate of absorption increases. Indications: superficial anesthesia: – skin during punctures and catheterization of blood vessels and superficial surgical interventions; – trophic ulcers of the lower extremities during surgical treatment (mechanical cleaning); – the mucous membrane of the genital organs before painful manipulations and for pain relief before injections of local anesthetics. Dosage regimen: for adults, for superficial anesthesia of intact skin, EMLA cream is applied to the skin under an occlusive dressing. For superficial anesthesia of trophic ulcers of the lower extremities, before their surgical treatment (mechanical cleaning), the cream should be applied in a thick layer to the ulcerative surface under an occlusive PVC dressing in a dose of 1–2 g/10 cm2 (no more than 10 g per procedure) for 30 minutes. If penetration of the drug into the ulcer tissue is difficult, the duration of application can be increased to 60 minutes. Mechanical cleaning of the ulcer should begin no later than 10 minutes. after removing the cream. For superficial anesthesia of the genital organs, before injections of local anesthetics, the cream should be applied in a thick layer to the skin for men at a dose of 1-2 g/10 cm2 for 15 minutes, for women - at a dose of 1 g/10 cm2 for 60 minutes. (Table 1). For superficial anesthesia of the mucous membrane of the genital organs during removal of condylomas and before injections of local anesthetics, 5–10 g of cream (depending on the area of the treated surface) is applied to the entire surface of the mucous membrane, including folds. Application time 5–10 minutes. The procedure should be carried out immediately after removing the cream. For children, for superficial anesthesia of intact skin before puncture of a vessel or superficial surgical interventions, EMLA cream is applied in a thick layer to the skin under an occlusive dressing. The dose of the drug should correspond to the surface being treated and should not exceed 1 g of cream per 10 cm2 (Table 2). For patients (especially children) with widespread neurodermatitis (atypical dermatitis), the application time should be reduced to 15–30 minutes. Side effect. Local reactions: often (>1%) – pallor, hyperemia and swelling at the site of application of the drug (due to the effect on superficial vessels); sometimes (<1%–>0.1%) – mild burning and itching immediately after applying the drug; rarely (<0.1%) - hemorrhagic rash or pinpoint hemorrhages, especially after prolonged application in children with widespread neurodermatitis or molluscum contagiosum. Systemic reactions: rarely (<0.1%) - allergic reactions (in severe cases - anaphylactic shock), methemoglobinemia in children. Contraindications to the use of EMLA cream are methemoglobinemia, premature babies born at less than 37 weeks of gestation, hypersensitivity to amide-type local anesthetics or any other component of the drug. It is possible to use EMLA during pregnancy and lactation according to indications and in recommended doses. Lidocaine and prilocaine are excreted in breast milk in quantities that do not pose a risk to the infant. Special instructions. Caution should be exercised when applying EMLA cream near the eyes as the drug causes irritation to the cornea. The cream should not be used if it can penetrate into the middle ear. The cream should not be applied to open wounds. Use in pediatrics. In children under 3 months of age. The safety and effectiveness of using EMLA cream is determined after applying a single dose. In children of this age, after applying the cream, a temporary increase in the concentration of methemoglobin in the blood may be observed for up to 13 hours, which has no clinical significance. The effectiveness of the cream in newborns during the procedure of taking blood samples from the heel has not been established. Overdose. Symptoms: stimulation of the central nervous system, in severe cases - depression of the central nervous system and cardiac activity. In some cases, children experienced the development of clinically significant methemoglobinemia. Application of 125 mg of prilocaine for 5 hours caused the development of moderate methemoglobinemia in a three-month-old child. Application of lidocaine at a dose of 8.6–17.2 mg/kg caused severe intoxication in newborns. Treatment: the unabsorbed part of the drug should be removed from the surface of the skin. If symptoms from the central nervous system (convulsions, central nervous system depression) occur, symptomatic therapy is indicated, incl. prescription of anticonvulsant therapy and, if necessary, mechanical ventilation. If methemoglobinemia develops, methylene blue should be used as an antidote. It is necessary to monitor the patient for several hours after starting treatment for intoxication due to the slow systemic absorption of the drug components. Drug interactions. When EMLA is used simultaneously with other local anesthetics and structurally similar drugs (including tocainide), the risk of systemic side effects may increase. With simultaneous use of the drug EMLA with drugs that induce the development of methemoglobinemia, drugs containing a sulfo group, an increase in the concentration of methemoglobin in the blood is possible. Storage conditions and periods. The drug should be stored out of the reach of children at a temperature not exceeding 30 °C; do not freeze. Shelf life – 3 years. In the Tushino Children's City Hospital in Moscow, in order to reduce the traumatic impact of pain on the child's body, over the past few years they have been using techniques that humanize the necessary painful medical procedures. Such techniques include the use of a local anesthetic in the form of EMLA cream during catheterization of peripheral veins. The purpose of this study is to evaluate the effectiveness of EMLA during superficial anesthesia of the skin before injections in children. The studies were carried out on 40 patients of both sexes aged from 1.5 to 15 years. The method of applying EMLA cream was the same in all cases. The cream was applied and evenly distributed at the site of the medical procedure and covered with the occlusive film included with the kit. The anesthesia procedure and subsequent removal of formations in children were carried out in the presence of parents and with their consent. The application time was about 60 minutes, after which the film was removed and the remaining cream was removed with a cotton swab, then the planned procedure was carried out. In 20% of children, blanching of the skin in the area where the drug was fixed was observed, which disappeared after 6 hours. 1 hour after applying the cream when the needle was inserted, no motor reaction to manipulation was noted. Subjectively, on a scale of verbal assessment of pain intensity – 0 (no pain). When examining respiratory rate, pulse, and blood pressure before and after the procedure, no significant changes were revealed. The duration of the anesthetic effect was 6 hours. No side effects were noted. Thus, the conducted studies show that the local anesthetic drug EMLA is effective when performing injections for the purpose of puncture and catheterization of peripheral veins in children. An important condition for adequate anesthesia is the correct technique for applying the cream, occlusive dressing and compliance with the recommended exposure time.
Literature 1. Badalyan L.O. Child neurology. M.: Medicine, 1984. pp. 306–329. 2. Bashkatova V.G., Vitskova G.Yu., Narkevich V.B. and others // Neurochemistry. 1996. T. 13, No. 2. P. 110–115. 3. Kalyuzhny L.V. Physiological mechanisms of regulation of pain sensitivity. M., 1984. P. 215. 4. Klipinina N.V. Some features of the perception and experience of pain by children: a psychologist’s view // Rus. Honey. magazine. 2007. T. 15. No. 1. 9 p. 5. Kukushkin M.L., Reshetnyak V.K. Mechanisms of acute pain and chronic pain syndromes // “Materia Medica”. 1998. No. 3. pp. 5–23. 6. Kryzhanovsky G.N. Determinative structures in the pathology of the nervous system. M., 1980. P. 360. 7. Tsibulyak V.N., Tsibulyak G.N. Trauma, pain, anesthesia. M., 1994. pp. 7–11. 8. Craig KD, Whitfield LF, Grunau RVE, Linton J., Hadjistavropoulos HD Pain in the preterm neonate: behavioral and physiological medicines // Pain. 1993. V. 52. P. 287–299. 9. Arendt–Nielsen L., Bjerring P. Depth and duration of skin analgesia to needle insertion after topical application of EMLA–cream // Med. 1989. V. 64. P. 173–177. 10. Crunau RVE, Johnston CC Craig KD Neonatal facial and cry responses to invasive and noninvasive procedures // Pain. 1990. V. 42. P. 295–305. 11. Coumer E., Karoubi P., Hanache A., Merbouche S., Mouchnino G., Leraillez J. Use of EMLA creams in a department or neonatology // Pain. 1996. V. 68. P. 431–434. 12. Coumer E., Hanache A., Karoubi P. et al. Using: of EMLA cream in 500 Neonates. Glasgow, Scotland: XV the European Congress of Perinatal Medicine. 1996. Abstract. 13. Coumer E., Hanache A., Karoubi P. et al. Problems cutanes application d'EMLA chez des prematures // Arch. Pediatric. 1997. V. 3. P. 239–290.
Emla drug interactions
EMLA cream may increase the formation of methemoglobin in patients receiving treatment with methemoglobin-inducing drugs (eg, sulfonamides). When using EMLA cream in high doses in patients receiving local anesthetics or drugs structurally similar to local anesthetics, such as tocainide, the risk of systemic additive effects must be considered. Specific studies of the interaction of the drug with local anesthetics and antiarrhythmic drugs belonging to class III have not been conducted, so caution is recommended when using them together.
Emla drug overdose, symptoms and treatment
If the recommended dosage regimen is followed, the development of systemic toxicity is unlikely. The probable symptoms of intoxication are the same as with the use of other local anesthetics: at the beginning, stimulation of the central nervous system, in severe cases, depression of the central nervous system and cardiac activity. Cases of clinically significant methemoglobinemia have been very rarely reported in children, as prilocaine in high doses can increase methemoglobin levels. Superficial application of 125 mg of prilocaine for 5 hours caused the development of moderate methemoglobinemia in a three-month-old child. Topical application of 8.6–17.2 mg/kg lidocaine led to the development of severe intoxication in infants. Severe neurological symptoms (convulsions, central nervous system depression) require symptomatic treatment: the use of mechanical ventilation and anticonvulsants. The antidote for methemoglobinemia is methylthionine. Due to the slow systemic absorption of the drug, the patient's condition must be monitored for several hours after the symptoms of intoxication have resolved.