Content
- 1 Gonadotropic hormones
- 2 Secretion of gonadotropic hormones 2.1 Regulation of GnRH secretion
- 2.2 Mechanism of action of GnRH
- 5.1 Diagnostic application
- 6.1 Pregnancy test
- 7.1 Female infertility
In what cases is a hCG injection recommended?
Doctors advise resorting to injections of the hormone after receiving test results for its levels. Typically, the introduction of effective drugs is recommended in the following cases:
- Dysmenorrhea,
- Problems with the ovaries
- Disadvantages of the ovulation process,
- Problems associated with the corpus luteum
- The prospect of miscarriage
- Inability of the body to bear a child,
- Therapy before in vitro fertilization,
- Formation of placental tissue.
Medicines containing hCG are used for reproductive procedures of various specifics, since the hormone has a positive effect on the functioning of the ovaries.
Gonadotropic hormones[edit | edit code]
The gonadotropic hormones of the pituitary gland include follicle stimulating hormone (FSH)
and
luteinizing hormone (LH)
.
Chorionic gonadotropin (CG)
also affects the gonads, but is synthesized by the placenta. These three hormones, together with thyroid-stimulating hormone (TSH), are similar in structure and form the family of glycoprotein hormones. All of them consist of two subunits: the α-subunit is the same for all, and the β-subunits differ and provide specific activity. Moreover, the β-subunits of all hormones are quite similar, with the exception of the β-subunit of hCG, which contains an additional section of 30 amino acid residues at the C-terminus and several additional carbohydrate residues. Carbohydrate residues increase the half-life (T1/2) of these hormones in serum and are also involved in their binding to receptors. In humans, the gene encoding the FSH β-subunit is localized in segment 11p13, and the LH β-subunit is located in segment 19ql2.32, adjacent to at least 7 genes encoding the hCG β-subunit. The gene encoding the α-subunit of these hormones is localized in the 6q21-q23 segment.
Secretion of gonadotropic hormones[edit | edit code]
Figure 56.4.
Hypohalamic-pituitary-gonadal system The regulation of the secretion of gonadotropic hormones is described in detail in the article - sex hormones. LH and FSH are synthesized by gonadotropic cells, which make up about 20% of the secreting cells of the adenohypophysis. HCG, which is found only in humans, primates and horses, is synthesized by syncytiotrophoblast cells. The synthesis of FSH and LH is stimulated by gonadoliberin and is regulated by feedback by sex hormones (Fig. 56.4 and 58.2).
Figure 58.2. Neuroendocrine regulation of the secretion of gonadotropic hormones in women.
Regulation of GnRH secretion[edit | edit code]
Gonadotropin-releasing hormone and its analogues
Gonadotropin-releasing hormone is a hormone that stimulates the synthesis and secretion of FSH and LH by gonadotropic cells of the adenohypophysis. GnRH is formed as a result of proteolytic cleavage of a polypeptide containing 92 amino acid residues, which is encoded by a gene located in the 8p21 segment. GnRH itself is a decapeptide with an amide group at the C-terminus and a pyroglutamic acid residue at the N-terminus (Table 56.3). GnRH secretion occurs in impulses as a result of the synchronized rhythmic activity of a group of neurons located in the region of the infundibulum nucleus. The activity of these neurons begins before birth and continues for about a year after birth; then it decreases significantly, probably as a result of the inhibitory effect of the central nervous system. Shortly before the onset of puberty, the inhibitory effect of the central nervous system decreases, and the frequency and amplitude of GnRH secretion impulses increase, especially strongly during sleep. As puberty develops, GnRH secretion continues to increase until it reaches adult levels. The pulsed nature of GnRH secretion is necessary for the normal synthesis and secretion of gonadotropic hormones, the secretion of which also occurs pulsed. Constant administration of GnRH leads to desensitization and a decrease in the number of GnRH receptors on the gonadotropic cells of the adenohypophysis. This phenomenon underlies the clinical use of long-acting GnRH analogues, which suppress the secretion of gonadotropins (see below). After their administration, the secretion of LH and FSH increases briefly, but then receptor desensitization occurs and secretion decreases.
Mechanism of action of GnRH[edit | edit code]
The GnRH receptor is a G-protein coupled receptor; its gene is located in segment 4q21. When GnRH or its analogues bind to the receptor, the Gq and GM proteins are activated, which, in turn, activate phospholipase C, which leads to an increase in intracellular calcium concentration. This ultimately stimulates the synthesis and secretion of LH and FSH. Although cAMP is not significantly involved in signal transduction from the GnRH receptor, activation of this receptor increases the activity of adenylate cyclase. GnRH receptors are also present in the ovaries and testes, but their physiological significance remains unknown.
Sex hormones also regulate the synthesis of LH and FSH, affecting the hypothalamus and, to a lesser extent, the pituitary gland. This regulation occurs differently in men and women and depends on age and the phase of the menstrual cycle. In women, low concentrations of estradiol and progesterone suppress the synthesis of gonadotropic hormones due to an opioid-mediated effect on neurons responsible for the impulse secretion of GnRH. However, a prolonged increase in estradiol concentration acts on the principle of positive feedback, leading to a preovulatory LH surge. In men, the synthesis of gonadotropic hormones is suppressed by testosterone, and this is partly due to its direct action, and partly due to its conversion to estradiol.
Inhibin is a peptide hormone produced in the gonads and plays an important role in regulating the secretion of gonadotropic hormones. It is synthesized by granular layer cells of the follicles in the ovaries and Sertoli cells in the testes in response to stimulation by gonadotropins and local growth factors. Inhibin acts directly on the pituitary gland, selectively suppressing the secretion of FSH, but not LG. Inhibin belongs to the same family of glycoproteins as transforming growth factor |3 and Müllerian duct regression factor.
Gonadotropins and antigonadotropins in gynecology
Authors : Mayorov M.V.
«Fortuna comprobat hominis consilium” (“Success confirms the correctness of plans”, lat.).
More than 70 years ago, German scientists Aschheim and Zondek provided convincing evidence of the gonadotropic function of the anterior pituitary gland. The effects of introducing the extract of the anterior lobe of the pituitary gland into follicle-stimulating and luteinizing soon followed. In 1932, Hohlweg and Junkmann, but not until 1960, McCann et al. identified LH-releasing factor in the rat hypothalamus. Subsequently, Schally and Guillemin established that gonadotropin-releasing hormone (GnRH) is a decapeptide and deciphered its structure.
As is known, in the anterior lobe of the pituitary gland two different gonadotropic hormones are formed, differing in chemical structure and immunological properties: follicle-stimulating hormone (FSH), or follitropin, luteinizing hormone (LH), or lutropin. In addition, adrenocorticotropic hormone (ACTH), or corticotropin, thyroid-stimulating hormone (TSH) or thyrotropin, growth hormone (GH), or growth hormone, as well as prolactin (PRL), which inhibits the production of gonadotropic hormones, are produced here. The relative molecular weight of FSH and LH in humans is approximately 32,000. These hormones can exist as monomers, dimers, and tetramers (10). The release of FSH, LH and PRL is regulated by releasing - and releasing - inhibitory factors of the hypothalamus.
The regulation of ovulation in women and spermatogenesis in men is the result of a rather complex interaction between the hypothalamus, adenohypophysis and sex hormones produced in the gonads. This system is controlled by three feedback mechanisms: 1. “long feedback” - the action of sex hormones on the hypothalamus is carried out through the mechanism of negative (estrogens, progesterone and testosterone) and positive (estrogens and progesterone) feedback; 2. “short feedback”, which determines the direct regulatory effect of sex hormones on the secretion of FSH and LH by the adenohypophysis; 3. “ultra-short feedback”, which is the regulation of the release of gonadotropin-releasing hormone (GnRH) under the influence of FSH and LH of the pituitary gland.
Apparently, the release of GnRH depends on dopamine contained in the nerve endings of the hypothalamus; in turn, GnRH acts on the FSH- and LH-secretory cells of the adenohypophysis. The secretory activity of the hypothalamus and pituitary gland is modulated differently by estrogens, progesterone and testosterone. It is believed that GnRH has a similar effect in men and women, with the exception of the cyclical nature of its release (in women), determined by the hypothalamus. The regulatory activity of GnRH is concentrated in two centers: tonic (in the ventromedial and arcuate nuclei) and cyclic (in the preoptic suprachiasmatic region). In men, the tonic center dominates, activating the pituitary gland and, as a result, the activity of the testicles, which causes spermatogenesis and testosterone synthesis.
In women, the absence of androgens appears to determine the appearance of the so-called “hypothalamic clock” necessary for the cyclic release of gonadotropins (i.e., the cyclic center dominates). It is sensitive to external and internal influences, in the presence of female sex hormones it is activated (or vice versa, suppressed) and indirectly stimulates the tonic center, causing an increase in the secretion of LH (mainly by the adenohypophysis) and subsequent ovulation. Isolated from the hypothalamus of pigs, sheep, and cattle, GnRH is a decapeptide; its functional parts in humans and pigs have the same structure.
Regulation of reproduction and gonadal function is carried out mainly by gonadotropic hormones secreted by the adenohypophysis - FSH, LH and PRL (12). FSH causes the proliferation of granulosa cells, stimulating the growth of follicles, LH activates the synthesis of androgens and, together with FSH, promotes ovulation. The secretion of FSH and LH is regulated by GnRH according to the feedback principle, carried out in a cyclical mode, but also depends on the level of estrogens and androgens.
According to the chemical structure, LH and FSH are glycoproteins, consisting of two polypeptide subunits α and β. The α subunit of these hormones is common to each glycoprotein and has the same amino acid sequence; The β subunit differs among glycoproteins in the sequence of its amino acids. It is the β subunit that is responsible for hormonal specificity. Separated from each other, both subunits are biologically inactive.
Like steroids, gonadotropins exert a biological effect on target tissues through the activation of specific receptors. However, unlike steroid hormones, gonadotropin receptors are associated with the membrane of target cells. Cell surface receptors for peptide glycoprotein hormones are proteins that are part of the structure of the cell membrane. Once bound to gonadotropin, membrane receptors stimulate the production of soluble intracellular messengers, which in turn mediate the cellular response (12).
According to modern concepts, regulators of FSH production, in addition to hypothalamic liberins, are inhibin and activin, produced by granulosa and luteal cells of the ovaries, as well as cytotrophoblast cells. The synthesis and release of FSH is also influenced by follistatin isolated from follicular fluid. There are three types of gonadotropin secretion: tonic, cyclic and episodic (pulsatile). Tonic (basal) secretion of gonadotropins is regulated by negative feedback, cyclic secretion by a positive feedback mechanism (with the participation of estrogens), pulsatile secretion by the activity of the hypothalamus and the release of gonadoliberins.
The development of the follicle in the first phase of the cycle occurs due to the tonic secretion of FSH and LH. An increase in estradiol secretion leads to inhibition of FSH formation. Consequently, FSH leads to the synthesis of estrogens in a certain follicle, which, by increasing the number of receptors for FSH, contribute to its accumulation (by binding to receptors), further maturation of the follicle and increased secretion of estradiol; other follicles undergo atresia at this time. The concentration of estradiol in the blood reaches a maximum in the blood during the preovulatory period, which leads to the release of large amounts of GnRH. This is followed by a peak of preovulatory increases in LH and FSH, stimulating rupture of the Graafian vesicle and ovulation.
LH is the main regulator of steroid synthesis in the ovaries. Receptors for LH are localized on luteal cells, and the effect of LH is mediated through stimulation of adenylate cyclase and an intracellular increase in cAMP levels. Enzymes involved in the biosynthesis of progesterone are activated, under the influence of LH in the ovaries the amount of cholesterol necessary for the synthesis of hormones increases, the activity of enzymes of the cytochrome P450 family and other enzymes involved in the synthesis of progesterone, as well as other steroids, increases. Thus, in the corpus luteum, under the influence of LH, the processes of steroidogenesis are enhanced.
Regulation of gonadotropin secretion is ensured by “short” and “ultra-short” feedback chains: an increase in the level of LH and FSH leads to inhibition of their synthesis and release, and an increased concentration of GnRH in the hypothalamus inhibits these processes. The catecholamines dopamine, adrenaline and norepinephrine have a stimulating effect on the release of GnRH, while cholecystokinin, gastrin, neurotensin, opioids and somatostatin have an inhibitory effect.
Gonadotropic hormones are used to induce ovulation, which is of great importance for the treatment of so-called functional female infertility, in the genesis of which anovulatory disorders occupy a significant place.
Before starting treatment, it is necessary to conduct appropriate hormonal studies to allow differential diagnosis of primary hypothalamic-pituitary insufficiency from primary ovarian insufficiency. For example, a normal or often low level of FSH suggests primary disorders in the hypothalamic-pituitary region. At this stage of diagnosis, it often becomes necessary to exclude a pituitary tumor. The inability to induce menstruation after treatment with high doses of estrogen may indicate a uterine form of amenorrhea.
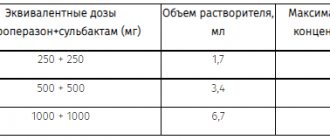
In medical practice, menopausal gonadotropin (menotropins), as well as its homologues (urofollitropin, follitropin alpha, follitropin beta) are used as drugs that have FSH activity - table. 1 . Menopausal gonadotropin is obtained from the urine of menopausal women. The drug with LH activity is human chorionic gonadotropin, obtained from the urine of pregnant women. All gonadotropins, as is known, are polypeptides that are destroyed by enzymes when taken orally, and therefore are effective only when administered parenterally.
Table 1 Gonadotropin preparations (2)
| Name | Forms release | Indications | Dosing |
| Human menopausal gonadotropins ( Human Menopausal Gonadotropins - HMG) Preparations: Menotropine, Pergonal, Humegon, Pergogrin | Each ampoule contains 75 IU of FSH and 75 IU of LH and 10 mg of lactose. Each ampoule comes with an ampoule of solvent (1 ml). | Infertility caused by anovulation and amenorrhea. Hypogonadotropic hypogonadism in men. | To induce the growth and development of follicles in the ovary and stimulate ovulation, 2 ml is administered intramuscularly once a day for 9 - 12 days, after which 10,000 IU of human chorionic gonadotropin (HCG) is administered once. The course can be repeated twice with a month's break (if stimulation has occurred, but pregnancy has not occurred). When repeating the course, the dose can be increased by 2 times (150 IU FSH and 150 IU LH); injections are carried out over 3 to 12 days, and then 10,000 hCG is administered once again. |
| Urofollitropin (Urofollitropin) Drugs Metrodin et al. | Powder for injection in ampoules of 75 IU and 150 IU FSH; Each ampoule comes with an ampoule of solvent (1 ml of 0.9% sodium chloride solution) | Stimulation of follicle maturation and ovulation in case of hypothalamic-pituitary dysfunction (amenorrhea, oligomenorrhea) Rhea) and to prepare the ovaries for in vitro fertilization (IVF). | 75 - 150 IU FSH intramuscularly or subcutaneously 1 time per day for 7 - 12 days; 24 - 48 hours after the last injection of Metrodin, 10,000 IU of hCG is administered. |
| Human chorionic gonadotropin (Human Chorionic Gonadotropin – HCG) Preparations: Profasi, Pregnyl LH, Choriogonin, Entromon, Follutein, Chorex, Gonic, Chorigon, Primogonyl, etc. | Powder for injection in ampoules of 500, 1000, 2000, 5000 and 10,000 IU HCG. An ampoule with a solvent (1 ml of 0.9% sodium chloride solution) is included. | Anovulation. Preparing the ovaries for IVF. Hypogonadotropic hypogonadism and cryptorchidism in men | To stimulate ovulation, it is administered subcutaneously or intramuscularly 24 to 48 hours after the last injection of Pergonal or Metrodin. |
Along with gonadotropic drugs, antigonadotropic drugs are widely used in medical practice - they inhibit the synthesis and secretion of gonadotropins directly or by inhibiting the release of the releasing hormone LH in the hypothalamus (2). Antigonadotropins are used, in particular, in the treatment of endometriosis (especially with concomitant infertility), benign breast tumors, premenstrual syndrome, gynecomastia and other diseases for which gonadotropin blockade is indicated.
A typical representative of the group of antigonadotropins is danazol (danol, danoval), which is a synthetic steroid similar in structure to ethisterone. The main mechanism of its action is the reversible suppression of the production of LH and FSH by the pituitary gland (in both women and men). Danazol does not have an estrogenic or gestagenic effect, but exhibits weak androgenic and anabolic activity and is especially active in endometriosis, premature puberty, gynecomastia, and benign breast tumors. It effectively reduces the size of the mammary glands and the feeling of tension in them in idiopathic or drug-induced gynecomastia, reduces or eliminates pain in severe cyclic mastalgia, even not relieved by analgesics.
For endometriosis, the initial daily dose is 400 mg, divided into 4 doses; then (if necessary) it can be increased to 600 - 800 mg, and the course of treatment should not exceed 6 months. Start of use - on the first day of the menstrual cycle. The drug is available in capsules of 100 and 200 mg. The side effects of danazol are partly due to its androgenic and anabolic effects (acne, acne, increased secretion of the sebaceous glands, deepening of the voice, hirsutism, clitoral hypertrophy, fluid retention in the body), as well as anti-estrogenic effects (hot flashes, emotional lability, bouts of profuse sweating, reduction in the size of the mammary glands), sometimes - headache, dizziness, tachycardia, nausea, hair loss, skin rash.
Danazol is contraindicated during pregnancy and porphyria; Particular caution should be exercised in the presence of kidney disease, liver disease, heart disease, diabetes mellitus (the need for insulin increases), as well as in cases of simultaneous use of anticonvulsants and anticoagulants. In many countries, danazol and its analogues are recognized as the main remedy used for endometriosis, as it has a fairly rapid therapeutic effect (relief of pain and elimination of other symptoms), in many cases contributing to the regression of endometriotic heterotopias.
The group of drugs that have an antigonadotropic effect includes gestrinone (nemestran), a synthetic steroid hormone, a derivative of 19-norsteroids, which has a strong antiprogestin effect. It not only reduces the release of gonadotropin, inhibiting the synthesis of steroids in the ovaries, but also exhibits a direct antagonistic effect directly in the endometriotic tissue in relation to estrogens and progesterone - by blocking their receptors. Gestrinone is used only for the treatment of patients with endometriosis; its characteristic side effects are due to androgenic effects. A transient increase in transferase activity (AST and ALT), libido disorders, nervousness and depression, changes in appetite, headache, and gastrointestinal disorders are also possible. Contraindicated in pregnancy, lactation, liver, kidney and heart diseases. Available in capsules of 2.5 mg, treatment begins on the first day of the menstrual cycle, 2.5 mg is prescribed 2 times a week (the second dose is on the 4th day of the cycle). The duration of treatment is 6 months.
In recent years, synthetic analogues of natural GnRH (Luliberin), in particular, goserelin acetate (Zoladex), have appeared on the pharmaceutical market in our country. By blocking the release of GnRH, goserelin leads to a decrease in the synthesis of estradiol and a decrease in its concentration in the blood. This helps to improve the course of endometriosis and even its regression, since the growth and development of endometriotic tissue are estrogen-dependent processes. There is also a slowdown in the progression of hormone-dependent forms of breast cancer and uterine fibroids.
The main indications for the use of GnRH analogues are: endometriosis, hormone-sensitive forms of breast cancer in premenopausal and menopausal women, symptomatic uterine fibroids (with contraindications to surgical treatment, or as preoperative preparation), as well as prostate cancer in men.
Characteristic side effects of goserelin include symptoms resulting from the “loss” of the action of sex hormones: “hot flashes”, bouts of profuse sweating, dryness of the vaginal mucosa, dyspareunia, headaches, emotional lability, depression, osteoporosis, and sometimes a decrease in the size of the mammary glands. The drug is contraindicated during pregnancy, lactation and childhood. It is produced in the form of a depot - a drug, a single dose of which (3.6 mg) is in a disposable syringe - applicator, injected subcutaneously, preferably into the anterior abdominal wall, 1 time every 28 days, for no more than 6 months.
Synthetic GnRH analogues leuprorelin acetate (Prostap), diferelin (triptorelin) and nafarelin acetate (sinarel) have a similar effect.
The latter drug is used only for endometriosis and is available in the form of a solution (0.002 g/ml) in 10 ml bottles (for 30 days of treatment). The daily dose (400 mcg) is administered intranasally 2 times a day - 200 mcg in the morning and evening; treatment should begin between the 2nd and 4th days of the menstrual cycle and continue for 6 months. Nafarelin acetate is contraindicated during pregnancy, lactation and hormone-dependent neoplasms of various localizations.
The use of gonadotropins and antigonadotropins in medical practice, of course, can significantly increase the effectiveness of treatment of many diseases, the success of treatment of which was previously quite problematic. However, effective treatment of these rather complex (and expensive!) drugs in often very complex patients requires deep knowledge from the doctor, as well as a certain courage and caution. For “Fortitudo circumsistitur hinc audacia, inde timiditate” (“Courage is found between reckless courage and timidity,” from Apuleius, Latin).
LITERATURE
1. Bodyazhina V.I., Smetnik V.P., Tumilovich L.G. Non-operative gynecology, Moscow, Medicine, 1990, 544 p. 2. Boroyan R. G. Clinical pharmacology for obstetricians and gynecologists, Moscow, MIA, 1999, 224 p. 4. Zilva J.F., Panell P.R. Clinical chemistry in diagnosis and treatment, trans. from English, Moscow, Medicine, 1988, 528 p. 5. Kokhanevich E.V. (ed.) Current issues in gynecology, Kyiv, Book-plus, 1998, 53 p. 6. Mayorov M.V. Endometriosis: a mysterious disease that challenges clinicians // Pharmacist, 2002, No. 18, p. 25 – 28 7. Mayorov M.V. Polycystic ovary syndrome: modern view // Pharmacist, 2002, No. 16, p. 39 – 41. 8. Malevich K. I., Rusakevich P. S. Treatment and rehabilitation for gynecological diseases, Minsk, Higher School, 1994, 368 p. 9. Powerstein K. J. (ed.) Gynecological disorders, trans. from English, Moscow, Medicine, 1985, 592 p. 10. Satoskar R. S., Bandarkar S. D. Pharmacology and pharmacotherapy, in 2 volumes, trans. from English, Moscow, Medicine, 1986. 11. Susloparov L. A. (ed.) Gynecology. Newest reference book, Moscow - St. Petersburg, EKSMO, 2003, 688 p. 12. Tatarchuk T.F., Solsky Ya.P. Endocrine gynecology (clinical essays), part 1, Kyiv, 2003, 300 p. 13. Taylor R.B. Difficult diagnosis, in 2 volumes, trans. from English, Moscow, Medicine, 1988. 14. Tronko M. D. (ed.). Standards for diagnosis and treatment of endocrine diseases, Kiev. 2005, 312 p. 15. Tits N. U. (ed.) Clinical evaluation of laboratory tests, trans. from English, Moscow, Medicine, 1986, 480 p. 16. Schambach H., Knappe G., Karol V. Hormone therapy, translated from German, Moscow, Medicine, 1988, 416 p. 17. Cry SJ Clinical uses of luteinizing hormone-releasing hormone (LHRH), Fertil. And Steril., 1983, 39, 577. 18. Groot - Wassink K. Gonadotropine und Gonadotropin - Releasing - Hormon, DDR-Med. Rep., 1981, 10, 531.
Consultations by Mark Veniaminovich Mayorov at ClubCom
published 08/11/2013 16:58 updated 30/11/2013 — Obstetrics, gynecology, mammology, Women's health
Mechanism of action of gonadotropic hormones[edit | edit code]
LH and hCG bind to the LH receptor (the gene for this receptor is located in segment 2p21), and FSH binds to its receptor (the gene for this receptor is located on the long arm of the 2nd chromosome). Both receptors are G protein coupled and have a large extracellular glycosylated domain responsible for hormone recognition. When the receptor binds to the ligand, the G protein activates adenylate cyclase, which leads to an increase in the concentration of cAMP. At a high concentration of the ligand, the receptors also activate protein kinase C through the Gq protein and increase the calcium concentration due to the activation of phospholipase C. All or almost all effects of gonadotropic hormones can be reproduced by introducing cAMP analogues into the cell, therefore the importance of protein kinase C and Ca2+ in intracellular signal transmission remains unclear.
Physiological effects of gonadotropic hormones[edit | edit code]
LH and FSH were named according to their effects on the ovaries; the function of these hormones in men was studied later. In men, LH acts on Leydig cells, stimulating the synthesis of androgens, mainly testosterone. Testosterone ensures sexual desire, the development of secondary sexual characteristics and spermatogenesis in the convoluted seminiferous tubules. FSH acts on Sertoli cells, stimulating their production of proteins and nutrients necessary for sperm maturation.
In women, the effects of FSH and LH are more complex. FSH stimulates the growth and development of follicles and also induces expression of the LH receptor gene on thekocytes and granular layer cells in the ovaries. In addition, FSH activates aromatase in the cells of the granular layer, stimulating the synthesis of estradiol. LH acts on thekocytes, stimulating the formation of androstenedione in them, the main precursor of estradiol in the ovaries of women of childbearing age. LH is also necessary for the rupture of the follicle during ovulation and for the synthesis of progesterone by the corpus luteum. Finally, the binding of LH to its receptor on granular layer cells increases the expression of the FSH receptor gene, which enhances the effect of the latter.
The importance of gonadotropic hormones in the regulation of the functions of the genital organs is clearly revealed by mutations in the genes of the hormones themselves or their receptors (Achermann and Jameson, 1999). Women with mutations in the FSH receptor genes or the β-subunit of FSH itself experience primary amenorrhea and infertility, their follicles do not mature, there are no corpora lutea and the mammary glands do not develop. These data, as well as the effectiveness of FSH in some forms of infertility (see below), eloquently indicate the important role of FSH in the functioning of the ovaries. In men with the same mutations, testicular size is reduced and oligozoospermia is observed, although in some cases fertility is preserved.
Only one case of a loss-of-function mutation of the LH β-subunit gene has been described: a 46-year-old man had no sexual development, Leydig cell hypoplasia and infertility were observed. His external genitalia were developed according to the male type, which is apparently explained by the synthesis of androgens under the influence of hCG during intrauterine development. Manifestations of loss-of-function mutations in the LH receptor gene in a male karyotype range from male hypogonadism to female-type development of the external genitalia and lack of sexual development. Probably, virilization of the external genitalia does not occur due to disruption of the action of both LH and hCG during intrauterine development. In women homozygous for mutant alleles of the LH receptor gene, primary amenorrhea or oligomenorrhea and infertility are observed, and histological examination reveals multiple ovarian cysts.
Mutations leading to permanent activation of the LH receptor occur predominantly in men and are inherited in an autosomal dominant manner. They lead to premature sexual development due to uncontrolled testosterone synthesis in the prenatal and prepubertal periods. Some of these mutations have a high risk of testicular tumors.
TESTOSTERONE
The bulk of testosterone (male sex hormone) is produced by the testes; a smaller amount is produced by cells of the reticular layer of the adrenal cortex and during transformation from precursors in peripheral tissues. In women, testosterone is formed during the process of peripheral transformation, as well as during synthesis in the cells of the inner lining of the ovarian follicle and the reticular layer of the adrenal cortex. Testosterone ensures in men the formation of the reproductive system according to the male type, the development of male secondary sexual characteristics during puberty, is responsible for maintaining sexual function (libido and potency), sperm maturation, development of the skeleton and muscle mass, stimulates the bone marrow, the activity of the sebaceous glands, modulates synthesis of b-endorphins (“hormones of joy”), insulin. In women, it is involved in the mechanism of follicle regression in the ovaries and in the regulation of the level of gonadotropic hormones of the pituitary gland. Only free testosterone dissolved in plasma is biologically active, which in the human body plays the role of a protein anabolic, that is, it stimulates protein synthesis. It is for this reason that men tend to be larger and more muscular than women. In many structures localized (located) in the skin, testosterone supplied with the blood determines the male type of hair (beard growth, etc.) and excess sebaceous secretions (seborrhea). Testosterone levels in men increase during puberty and remain high until the age of 60 on average. During the day, the concentration of the hormone in the blood plasma fluctuates (max. - in the morning, min. - in the evening). In women, the maximum concentration of testosterone is determined in the luteal phase and during ovulation. In pregnant women, it increases by the third trimester, exceeding almost 3 times the concentration in non-pregnant women. Indications for the analysis: In both sexes: infertility, baldness, acne, oily seborrhea, adrenal tumors. In women: hirsutism, anovulation, amenorrhea, oligomenorrhea, dysfunctional uterine bleeding, miscarriage, polycystic ovary syndrome, uterine fibroids, endometriosis, breast neoplasms, hypoplasia (underdevelopment) of the uterus and mammary glands. In men: impaired potency, decreased libido, male menopause, primary and secondary hypogonadism, chronic prostatitis, osteoporosis. An increase in testosterone levels in the blood may indicate premature puberty (in boys), adrenal hyperplasia, or tumors that produce sex hormones. Testosterone levels are usually reduced in Down syndrome, renal, liver failure, and gonadal failure. Preparation for the study: on the eve of the study, it is necessary to exclude physical activity (sports training) and smoking. In women, the analysis is performed on days 6-7 of the menstrual cycle, unless other dates are indicated by the attending physician.
The use of GnRH and its analogues[edit | edit code]
In table 56.3 lists synthetic analogues of GnRH used in the clinic. Synthetic GnRH is called gonadorelin. Substitution of the amino acid residue at position 6 protects GnRH analogues from proteolysis, and substitution of the C-terminal residue increases affinity for the receptor. Such analogues have greater activity and a longer action compared to native GnRH, whose T1/2 is only 2-4 minutes.
GnRH antagonists, unlike their long-acting analogues, do not cause a transient increase in serum gonadotropin levels. Modern GnRH antagonists do not appear to lead to local and systemic histamine release and anaphylactic reactions, which limited the use of the first drugs in this group. Two GnRH antagonists, gairelix and cetrorelix, are used in in vitro fertilization to suppress the LH surge during ovarian stimulation. Ganirelix is available in the US, while Cetrorelix is only available in Europe. Theoretically, rapid suppression of LH surge allows for better control of ovarian stimulation (see below) and thereby shortens the artificial insemination cycle. However, the practical value of these drugs in artificial insemination remains to be clarified in clinical trials.
Diagnostic application[edit | edit code]
One of the gonadorelin preparations (gonadorelin hydrochloride) is used for the differential diagnosis of pituitary and hypothalamic lesions in secondary hypogonadism. Serum LH levels are measured before administration of gonadorelin at a dose of 100 μg subcutaneously or intravenously and within 2 hours (15, 30, 45, 60 and 120 minutes) after administration. An increase in LH secretion indicates the presence of functioning gonadotropic cells of the adenohypophysis. With prolonged deficiency of GnRH, the sensitivity of gonadotropic cells to it may decrease, so a low LH level during a test with GnRH does not necessarily indicate damage to the pituitary gland. The GnRH test can also be used to differentiate between true (associated with GnRH secretion) and false precocious puberty.
Infertility treatment[edit | edit code]
Another gonadorelin drug (gonadorelin acetate) is used for reproductive disorders associated with GnRH deficiency. The drug is administered intravenously using a special infusion pump in a pulse mode that simulates physiological secretion, 2.5 mcg every 60-90 minutes. If ovulation does not occur, the dose administered per pulse can be gradually increased to 10 mcg. The principles of administration and dosage are specified in the manufacturer's instructions. Gonadorelin is less likely than gonadotropic hormones to cause multiple pregnancies. When treating with gon-dorelin, it is not necessary to so carefully monitor the level of estrogen in the serum and perform ultrasound of the ovaries. Adverse reactions occur rarely; most often phlebitis develops at the injection site. When treated with gonadorelin, the physiological secretion of sex hormones is restored in women, the menstrual cycle is established and ovulation occurs. However, due to technical complexity, this treatment method is used only in specialized clinics (Hayes et al., 1998).
In case of infertility in men, gonadorelin can be used to achieve testicular growth, normal secretion of sex hormones and spermatogenesis. However, the method is relatively expensive and requires constant wearing of an infusion pump. In addition, gonadorelin is not approved by the FDA for the treatment of male infertility. Therefore, preference is usually given to gonadotropic hormones.
Long-acting GnRH analogs are also used for ovulation induction. These drugs suppress the preovulatory LH surge and prevent luteinization of the nonovulating follicle. Several treatment regimens have been developed in which GnRH analogs are administered long-term or short-term together with gonadotropic hormones that ensure follicle maturation (see below), and then hCG is prescribed to induce ovulation (Lunenfeld, 1999).
Suppression of the secretion of gonadotropic hormones[edit | edit code]
As already mentioned, long-acting GnRH analogues lead to desensitization of GnRH receptors, as a result of which the secretion of gonadotropic and, accordingly, sex hormones is sharply reduced. This “medical castration” has proven to be very convenient in cases where it is necessary to reduce the production of sex hormones. An obvious indication for such treatment is true precocious puberty in children, in which long-term administration of GnRH analogues is very effective and is almost not accompanied by adverse reactions.
Long-acting GnRH analogs are used for the palliative treatment of hormone-dependent tumors (for example, prostate or breast cancer); Since at the beginning of treatment these drugs sharply increase the secretion of sex hormones, at the same time drugs are prescribed that disrupt the synthesis or block the action of sex hormones. In addition, long-acting GnRH analogs are used for other hormone-dependent diseases (endometriosis, uterine fibroids and acute intermittent porphyria). Finally, for medical castration in mental disorders such as pedophilia, when the risk of non-compliance with medical prescriptions is very high, goserelin is especially convenient, which is administered at a dose of 10.8 mg subcutaneously once every 3 months.
Long-acting GnRH analogues are generally well tolerated and cause quite predictable side effects due to disruption of the synthesis of sex hormones: hot flashes, vaginal dryness, atrophic vaginitis, decreased bone density. In this regard, for diseases such as endometriosis and uterine fibroids, treatment is usually continued for no more than 6 months or additional estrogens are prescribed to maintain bone density.
LH (luteotropic hormone)
In women, luteotropic hormone stimulates the synthesis of estrogen; regulates the secretion of progesterone and the formation of the corpus luteum. In men, by stimulating the formation of sex hormone binding globulin (SHBG), it increases the permeability of the seminiferous tubules to testosterone. This increases the concentration of testosterone in the blood plasma, which promotes sperm maturation. In turn, testosterone re-inhibits the release of LH. The release of the hormone is pulsating in nature and depends in women on the phase of the ovulation cycle. During puberty, LH levels increase, approaching values typical for adults. In the menstrual cycle in women, the peak concentration of LH occurs at ovulation, after which the level of the hormone drops and remains throughout the luteal phase at lower values than in the follicular phase. During pregnancy the concentration decreases. During the postmenopausal period, the concentration of LH increases, as does FSH (follicle-stimulating hormone). In women, the concentration of LH in the blood is maximum in the period from 12 to 24 hours before ovulation and is maintained throughout the day, reaching a concentration 10 times higher compared to the non-ovulatory period. In men, LH levels increase by 60-65 years. Indications for the analysis: hirsutism, decreased libido and potency, anovulation, oligomenorrhea, amenorrhea, infertility, dysfunctional uterine bleeding, miscarriage, premature sexual development or delay, sexual infantilism, endometriosis, monitoring the effectiveness of hormone therapy. An increase in LH levels is observed with insufficient function of the gonads, polycystic ovary syndrome, pituitary tumors, renal failure, and gonadal atrophy in men after testicular inflammation. A decrease in LH levels occurs with secondary amenorrhea; hypofunction of the pituitary gland and hypothalamus, genetic syndromes, anorexia nervosa, polycystic ovary syndrome, luteal phase deficiency, surgical interventions. Preparation for the study: 3 days before taking blood, you must avoid sports training. 1 hour before blood collection - smoking. Immediately before taking blood, you need to calm down. Blood is drawn on an empty stomach, lying down. The analysis is done on days 6-7 of the menstrual cycle, unless other dates are indicated by the attending physician.
Diagnostic use of gonadotropic hormones[edit | edit code]
Pregnancy test[edit | edit code]
HCG is present in large quantities in the blood and urine of pregnant women, so immunochemical detection of the β-subunit of hCG can be used as a pregnancy test. Qualitative determination of the β-subunit of hCG in urine is the basis of home pregnancy tests sold over the counter in the United States. These tests can quickly detect pregnancy within a few days after a missed period.
Quantitative determination of the concentration of hCG in plasma for clinical and scientific purposes is carried out using RIA. This is usually done to assess the progression of pregnancy, or if an ectopic pregnancy, hydatidiform mole or choriocarcinoma is suspected.
Determining the time of ovulation[edit | edit code]
Ovulation occurs 36 hours after the onset of the LH surge and 10-12 hours after its peak concentration is reached. Therefore, the concentration of LH in the urine can predict the time of ovulation. Kits are produced for home use that allow semi-quantitative assessment of LH levels in urine using specific antibodies. The test is performed every 12 or 24 hours starting from the 11th day of the cycle (with a 28-day cycle) and the expected time of ovulation is determined by the increase in LH levels. This makes it possible to increase the likelihood of conception by choosing the right time for sexual intercourse.
Diagnosis of reproductive function disorders[edit | edit code]
Quantitative measurement of FSH and LH levels in plasma using the RIA method (based on the level of β-subunits) is used to diagnose certain reproductive disorders. Low or undetectable levels of FSH and LH indicate secondary hypogonadism and damage to the pituitary gland or hypothalamus. In primary hypogonadism, the levels of these hormones, on the contrary, are high. With amenorrhea in women or delayed puberty in men and women, determining the level of FSH and LH makes it possible to distinguish damage to the gonads from damage to the hypothalamic-pituitary system.
The level of FSH on the 3rd day of the menstrual cycle can be used to judge fertility. Even with a normal menstrual cycle, an FSH level of 15 IU/ml or more indicates low fertility, and the success of artificial insemination is unlikely (see below).
The hCG test is used in men to assess the function of Leydig cells based on the degree of stimulation of testosterone synthesis. This test is used if there is a suspicion of dysfunction of Leydig cells (for example, with delayed sexual development). Serum testosterone levels are determined after several injections of hCG. Reduced secretion of testosterone indicates a pathology of Leydig cells, and normal secretion indicates damage to the hypothalamic-pituitary system.
FSH (follicle stimulating hormone)
FSH is a pituitary hormone that regulates the functioning of the gonads. In men, it is secreted constantly evenly, in women - cyclically, increasing in the first phase of the menstrual cycle. FSH promotes the formation and maturation of germ cells: eggs and sperm. The egg in the ovary grows as part of a follicle consisting of follicular cells. These cells, during the growth of the follicle, under the influence of FSH, synthesize female sex hormones - estrogens, which, in turn, suppress the release of FSH (negative feedback principle). Indications for the analysis: decreased libido and potency, infertility, anovulation, oligomenorrhea, amenorrhea, dysfunctional uterine bleeding, miscarriage, premature sexual development or delay, polycystic ovary syndrome, endometriosis, growth retardation, chronic inflammation syndrome of the internal genital organs. An increase in FSH levels may indicate insufficient function of the gonads, a pituitary tumor, primary hypogonadism (in men), ovarian wasting syndrome (in women), renal failure, dysfunctional uterine bleeding and other conditions. The analysis will show a decrease in FSH levels with hypofunction of the pituitary gland or hypothalamus, pregnancy, secondary amenorrhea, polycystic ovary syndrome, hyperprolactinemia. Preparation for the study: The analysis is done on the 6-7th day of the menstrual cycle, unless other dates are indicated by the attending physician. 3 days before taking blood, you must avoid sports training. 1 hour before blood collection - smoking. Immediately before taking blood, you need to calm down. Blood is taken from a vein on an empty stomach, lying down.
Therapeutic use of gonadotropic hormones[edit | edit code]
Initially, gonadotropic hormones for clinical use were obtained from human pituitary glands (cadaveric material) and from the urine of women. Pituitary preparations are not currently used due to the risk of transmission of the causative agent of Creutzfeldt-Jakob disease. Several drugs are obtained from urine. HCG, which is similar in action to LG, is obtained from the urine of pregnant women. The drug menotropin is obtained from the urine of postmenopausal women, which contains approximately equal amounts of LH and FSH, as well as other urine proteins. Since menotropin is relatively poorly purified, it is administered intramuscularly to avoid allergic reactions. Another drug, urofollitropin, is purified FSH obtained by removing LH by immunological methods. Ultra-highly purified urofollitropin is obtained using monoclonal antibodies to FSH. It is purified so much that it can be administered subcutaneously.
Using genetic engineering methods, a mammalian cell line was obtained that synthesizes the a- and p-subunits of FSH. The resulting recombinant FSH is close in the nature of glycosylation to native FSH. Today, two recombinant FSH preparations are produced - follitropin A and follitropin P; their carbohydrate structures are slightly different. Both of these drugs are administered subcutaneously because they are much purer than urine-derived drugs and are much better standardized. Recombinant drugs are much more expensive than native ones, but there is no evidence yet that they are more effective or less likely to cause adverse reactions such as ovarian hyperstimulation syndrome. It is likely that in the future, using genetic engineering methods, it will be possible to obtain analogues of gonadotropic hormones with a longer action and with higher activity.
Female infertility[edit | edit code]
About 10% of married couples of childbearing age suffer from infertility. Gonadotropin hormones are increasingly used to treat infertility (Vollenhoven and Healy, 1998); They are often used for artificial insemination. Although the main indication for their use is chronic anovulation as a result of secondary hypogonadism (group 1 according to the WHO classification), gonadotropic hormones are also used to induce ovulation in polycystic ovary syndrome (group II according to the WHO classification) in cases where clomiphene is ineffective (Chapter 58 ). In addition, gonadotropic hormones are used for infertility against the background of normal ovulation, although in these cases treatment with clomiphene is first tried. Doctors with sufficient experience in treating infertility and endocrine disorders should prescribe gonadotropic hormones.
In chronic anovulation, administration of FSH alone induces ovulation in most cases. Typically, FSH is prescribed at a dose of 75 IU/day during the first 6-7 days of the cycle, after which the number and size of maturing follicles are assessed using vaginal ultrasound. Ultrasound is usually performed every 2-3 days. Follicle maturation is considered adequate if a follicle with a diameter of 18 mm is detected. If three or more follicles with a diameter of more than 16 mm are detected, FSH is discontinued due to the risk of ovarian hyperstimulation syndrome (see below) and barrier methods of contraception are used to prevent multiple pregnancies. The concentration of estradiol is also determined, which should be in the range of 500-1500 pg/ml. Lower values indicate insufficient ovarian stimulation, while higher values indicate the risk of ovarian hyperstimulation syndrome. If ovarian stimulation is insufficient, the dose of FSH can be increased to 150 IU/day.
To complete follicle maturation and induce ovulation, hCG is prescribed the day after FSH is discontinued at a dose of 5,000–10,000 IU. When treated with gonadotropic hormones, multiple pregnancies develop in 10-20% of cases, which is associated with the development of several tertiary follicles and the release of several eggs.
Gonadotropic hormones are used in artificial insemination, including in vitro fertilization and microinjection of sperm into the cytoplasm of the egg. With the help of FSH, the development of follicles is stimulated, and for their final maturation, hCG is prescribed, after which mature eggs are surgically removed from the tertiary follicles. Then, in vitro fertilization of the resulting eggs is performed using sperm or microinjection of sperm into the cytoplasm of the egg. The embryos are transferred into the uterus or fallopian tube. With artificial insemination, the risk of multiple pregnancy depends on the number of embryos transferred.
In addition to multiple pregnancies and its possible complications, the main side effect of gonadotropins is ovarian hyperstimulation syndrome. This syndrome is characterized by rapid accumulation of fluid in the peritoneal cavity, pleural cavity and even the pericardium. It is believed that the cause of this syndrome is the release of a substance by the ovaries that increases vascular permeability. Ovarian hyperstimulation syndrome is characterized by bloating, abdominal pain, nausea, vomiting, diarrhea, severe ovarian enlargement, shortness of breath and oliguria. Ovarian hyperstimulation syndrome can be complicated by hypovolemia, fluid and electrolyte disturbances, hemoperitoneum, ARDS, thromboembolism and liver dysfunction. If the development of this syndrome is suspected, hCG is not prescribed.
Some studies have suggested that gonadotropins increase the risk of ovarian cancer, but this remains unproven. It is important to note that ovarian stimulation with FSH and menotropin does not increase the risk of malformations in children born from stimulated eggs.
Male infertility[edit | edit code]
Treatment with gonadotropic hormones can be effective if the cause of infertility is their deficiency. Since gonadotropic hormones are relatively expensive, and long-term use can lead to resistance to them, sexual development is usually stimulated with androgens, and gonadotropins are used later to achieve fertility itself.
Treatment, as a rule, begins with the administration of hCG in a dose of 1000-5000 IU intramuscularly 3 times a week until the synthesis of sex hormones is normalized, as judged by clinical signs and plasma testosterone levels. After this, the dose of hCG is reduced to 2000 IU 2 times a week and menotropin is additionally administered at a dose of 75-150 IU LH and FSH 3 times a week. The most common side effect of gonadotropins is gynecomastia; it occurs in almost every third patient and is probably associated with increased production of estrogen: It takes approximately 6 months for the testicles to mature, and to establish full spermatogenesis, treatment must be continued for up to 2 years. After the onset of spermatogenesis or its resumption (in case of secondary hypogonadism that occurs after puberty), maintenance therapy with hCG is sufficient for sperm production. As already mentioned, recombinant gonadotropins are likely to play an increasingly important role in the treatment of infertility.
Cryptorchidism[edit | edit code]
With cryptorchidism, one or both testicles do not descend into the scrotum. In full-term newborn boys, it occurs in 3% of cases, but with age its prevalence decreases markedly. With cryptorchidism, spermatogenesis is disrupted and the risk of testicular germ cell tumors increases. Therefore, it is believed that the removal of the testicle into the scrotum should be done as early as possible, usually at the age of one year and in any case before 2 years. Since testicular descent is stimulated by androgens, hCG can be used in the absence of an anatomical obstruction. Usually 6 injections of hCG, 500 IU/m2 IM, are prescribed every other day. If such treatment is ineffective, orchiopexy is performed.
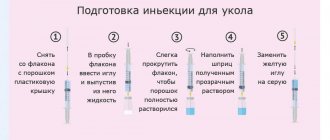
Carrying out an HCG injection
Intramuscular administration of the drug involves the possibility of self-injection, if the woman has the necessary experience and knowledge, but can also be carried out in a medical institution. You can also call a nurse to your home. It is worth taking into account that hCG injections are carried out in a course, so it is worth preparing for therapy in advance.
The hCG injection is given in the stomach. For many women, this is a frightening fact, since many are accustomed to injections into the buttock and do not really understand the meaning of such prescriptions. An injection in the stomach is more effective. To carry it out, measure a distance of two fingers from the navel to the left or right. Then, using your fingers, the skin of the abdomen is collected into a small fold and a needle is inserted into it. It does not penetrate the skin completely. The most relevant tool will be an insulin needle, which is used for injections for diabetes. The needle must first be sterilized in an alcohol solution.
The dosages of drugs recommended for injection may vary depending on medical prescriptions and the individual situation. The most popular dosages of the substance that can be purchased at the pharmacy are standards of 500, 1,000, 1,500, 5,000 and 10,000 units.
Depending on the indications, different numbers of hCG injections may be recommended, aimed at creating the necessary conditions for pregnancy. The standard dose of the drug for each use is considered to be 5-10 thousand units. Assessing the change in the situation, the doctor may prescribe a lower dose after several injections. When carrying out hormonal therapy, the size of the follicles is taken into account so as not to motivate excessive stimulation of the ovaries.
The main indication for hCG injection is diagnosed infertility. Some time after hormonal therapy, an ultrasound is performed, which makes it possible to clarify the presence of positive changes. If expectations are met, an hCG injection is given to stimulate ovulation. The positive effect of the hormone also lies in the fact that it does not give the follicles the opportunity to regress.
When stimulating superovulation as part of the reproductive program, the patient is administered a single dose of about 10 thousand units of human chorionic gonadotropin, which ensures the growth of several follicles at once, which will subsequently become eggs. 36 hours after the injection, you can select an egg that best meets the requirements for artificial conception.
If doctors diagnose a threat of miscarriage, the maximum dose is administered through an hCG injection, and then 5 thousand units of the hormone are injected twice a week. In order for hormonal therapy to remain relevant and effective, it must begin before the eighth week of pregnancy, and its end should be scheduled closer to the 14th week of pregnancy.