Risperidone is one of the popular and effective antipsychotic drugs (neuroleptics). The active substance itself is a benzisoxazole derivative. This medicine was first approved for use in 1993. Used in psychiatric and neurological practice. It is usually prescribed for all kinds of productive disorders, for example, hallucinations, delusions, etc. In addition, the drug reduces irritability and auto-aggression.
One of the advantages of Risperidone is that with its use the rate of elimination of mental and psychosomatic symptoms is twice as high as with the use of typical antipsychotics. In addition, this treatment appears to be safer.
Method of action
After oral administration, the drug is completely absorbed in the gastrointestinal tract and quickly distributed throughout the body. In this case, food does not affect the process in any way. Thanks to this, it is possible to achieve the optimal concentration of the substance in the plasma - it corresponds to the dose used.
A week after taking the drug, it is almost completely eliminated from the body - 70% is excreted in urine, 14% in feces. However, it is worth considering that in some situations slow elimination may occur. Most often this is due to poor functioning of the liver and kidneys (in old age, with renal failure, etc.).
Increasing the quality and accessibility of medical care and improving the healthcare system lead to an increase in life expectancy and, accordingly, an increase in the proportion of older people in the population structure. As life expectancy increases, the incidence of cognitive impairment and dementia increases. Thus, according to B. Plassman et al. [49], the prevalence of dementia among elderly people ranges from 5.0% in the age group 71–79 years to 37.4% in those aged 90 years and older.
Dementia is defined as an acquired decline in intellectual function. Impairment of intellectual functions in dementia is characterized by simultaneous impairment of memory, speech, attention, praxis, gnosis and more subtle functions, such as the ability to make decisions, plan and control one’s actions. Dementia limits the patient’s ability to self-care, impairs his everyday independence and social adaptation, and makes him incapable of professional activity. The relevance of the problem of dementia is steadily growing due to its high prevalence, the disabling course of the disease and the high economic costs of treatment and care for patients who, in the later stages of the disease, require lifelong hospitalization in specialized institutions (boarding schools for chronically mentally ill patients). Dementia most often occurs as a result of degenerative, atrophic and vascular lesions of the brain, according to which dementia of the Alzheimer's type, vascular dementia and mixed (Alzheimer's-vascular) dementia are distinguished.
Along with intellectual deficit, more than 50% of patients with dementia experience psychotic symptoms (delusions and hallucinations) and behavioral disorders (aggression, agitation, agitation, irritability, activity disturbances, apathy, disinhibition, etc.), as well as affective disorders (depression, anxiety , phobia) [44]. This correlates with the data of the study by S. Bergh et al. [12], which found that 91.7% of patients had at least one of the symptoms of mental and behavioral disorders, such as irritability (in 63.5%), agitation (in 51.0%), disinhibition (in 37.8%) and depression, and delusional disorders, agitation and irritability were persistent and constant.
As the duration and severity of dementia increases, the incidence of associated mental, behavioral and neurotic disorders increases. Thus, according to B. Plassman et al. [49], the prevalence of dementia in the United States among persons over 71 years of age is 13.9%, and 70% of these patients have symptoms of psychosis within 7 years of diagnosis. It was noted that during each subsequent year of the disease, patients experienced an increase in the total number of hallucinatory-delusional incidents: 20.1% in the 1st year, 36.1% in the 2nd, 49.5% in the 3rd and 51. 3% in the 4th [18, 46, 57].
An analysis of the literature devoted to the problem of dementia shows that researchers pay the greatest attention to cognitive disorders, and much less attention is paid to psychotic and behavioral disorders, although these disorders lead to a more rapid development of cognitive deficits, disruption of normal functioning and social behavior, leading to a deterioration in the quality of life and prognosis [46]. Psychotic and behavioral disorders, rather than intellectual-mnestic decline, often create the greatest difficulties for caregivers of patients with dementia and lead to an increase in the number of hospitalizations and, accordingly, an increase in the cost of therapy.
To determine the strategy and tactics of therapy, all specialists have recognized the need to conduct a full diagnostic examination of patients with dementia accompanied by psychotic disorders. It is necessary to determine the etiology of the syndrome of dementia and mental disorders and exclude any other causes, such as tumorigenesis or the development of drug-induced confusion.
The goals of therapy for patients with dementia with psychotic and behavioral disorders are the most complete relief of psychopathological symptoms, preservation and, if possible, improvement of cognitive functions. Considering that elderly patients, as a rule, suffer from comorbid somatic pathology and in most cases they are forced to take combination therapy, as well as the fact that the elderly often have impaired liver and kidney functions and, as a result, the level of drug metabolism and the processes of excretion of metabolites are reduced medications, when choosing therapy, the doctor must first of all be guided by the safety of the prescribed drugs and their combinations.
For the treatment of non-cognitive disorders in patients, various groups of psychotropic drugs are used: antipsychotics, antidepressants, tranquilizers, anticonvulsants, etc. Since the 50s, neuroleptics have been and remain the main means for correcting psychoses and behavioral disorders in dementia. The source of restoration and improvement of cognitive functions in patients using antipsychotics is their ability to reduce positive, negative and other symptoms.
A number of studies [8, 11, 16, 24, 35] have found that when positive and negative symptoms are relieved, cognitive functions are restored and improved. An important factor influencing the dynamics of cognitive functions is also the severity of extrapyramidal symptoms that arise during antipsychotic therapy, and, as a consequence, the need to use anticholinergic drugs.
First generation antipsychotic drugs, or typical neuroleptics, have been actively used in patients for many decades. Their disadvantage is certain side effects that develop in almost 90% of patients [47].
In patients with cognitive disorders and dementia, the most undesirable side effects are anticholinergic side effects, which can worsen cognitive impairment, which traditional antipsychotics themselves have little effect on. Even relatively short-term use of traditional antipsychotics with a pronounced anticholinergic effect may be accompanied by an increase in cognitive impairment; Of course, these disorders are aggravated by the use of correctors associated with extrapyramidal disorders [13, 36, 37, 40, 43, 51, 52, 55, 61]. When using antipsychotics, other undesirable anticholinergic effects may be observed, such as dry mouth, tachycardia, accommodation disturbances, impaired urinary excretion and urinary retention, constipation, orthostatic hypotension, etc. These side effects complicate the course of the underlying disease, leading to increased cognitive impairment and affective disorders, which is accompanied by worsening social maladjustment of patients. It can be stated with regret that effective and safe doses of first-generation antipsychotics for use in elderly patients with dementia have not yet been determined. It can only be noted that many specialists prefer the use of low doses.
A more modern group of drugs for the treatment of psychotic and behavioral disorders are atypical antipsychotics. Like typical antipsychotics, they block D2 receptors and are able to block serotonergic receptors such as 5-HT2, which determines their effect not only on positive but also negative symptoms, as well as on cognitive function. These drugs are more effective and safe, and are less likely to cause extrapyramidal symptoms such as parkinsonism and tardive dyskinesia [57].
It is important to emphasize that the use of atypical antipsychotics is accompanied by a lower risk of cerebrovascular disorders [21, 30, 41].
Of particular interest in this regard are two retrospective studies [23, 33], which compared the incidence of cerebrovascular disorders in elderly patients receiving atypical and typical antipsychotics.
A large number of studies [1–6, 13, 15, 17, 20, 25, 27–29, 31, 36–40, 42, 43, 50–52, 54–56, 58, 60–62] have found that that the use of atypical antipsychotics improves cognitive function. Thus, risperidone promotes the restoration of such cognitive functions as visual memory, praxis, gnosis, verbal, non-verbal and verbal-logical thinking; amisulpride affects praxis, especially through kinesthetic, kinetic and target components; quetiapine improves verbal and verbal-logical thinking, praxis, neurodynamics, voluntary regulation of activity, olanzapine affects auditory-verbal and visual memory, with a somewhat delayed effect on praxis; sertindole improves reaction time, working memory, executive function (problem-solving behavior).
One of the most well-studied atypical antipsychotics is risperidone. The drug acts mainly on the transmission of excitation by dopamine (D2) and serotonin (5-HT2) receptors [10]. In doses not exceeding average therapeutic ones, risperidone has no effect on the cholinergic system; during therapy with the drug, anticholinergic side effects are extremely rare, and the risk of sedation is minimal, this is due to the low tropism of the drug to the M1 and H1 receptors, respectively. It has been established that risperidone exhibits the lowest affinity for acetylcholine receptors compared to other atypical antipsychotics [26].
The effectiveness of risperidone in the treatment of psychotic and behavioral disorders in patients with dementia has been studied since the early 90s, but this issue remains poorly understood. A small number of foreign studies have shown a more pronounced effect of risperidone on psychotic and behavioral disorders in comparison with placebo and haloperidol, but without taking into account the effect of the drug on cognitive deficits [14, 19, 34, 59]. In one study [34], 625 patients with Alzheimer's disease, vascular and mixed dementia were treated, and 95% of patients suffered from severe dementia with psychotic and behavioral disorders (psychosis, aggression, agitation, etc.). Patients were randomized to matched groups and received placebo or 0.5, 1, or 2 mg/day risperidone for 12 weeks. It was confirmed that risperidone at a dose of 0.5, 1 and 2 mg/day was more effective in reducing symptoms of psychosis and aggressiveness compared to placebo (p=0.02, p=0.005 and p<0.001). Side effects such as drowsiness, extrapyramidal symptoms, minor peripheral edema were observed in a small number of patients; the incidence of extrapyramidal symptoms did not differ significantly between risperidone 1 mg/day and placebo. The researchers concluded that risperidone was more effective than placebo on symptoms of psychosis and aggressive behavior in patients with severe dementia (with a dose of risperidone 1 mg/day being adequate for most patients). In the work of H. Brodaty et al. [14] in 345 patients with dementia combined with psychotic and behavioral symptoms, similar results were obtained during 12 weeks of treatment with risperidone (average daily dose 0.95 ± 0.03 mg). It was concluded that low-dose risperidone therapy, compared with placebo, resulted in greater regression of the full range of symptoms of psychosis associated with dementia [45]. The incidence of extrapyramidal symptoms did not differ significantly between risperidone and placebo - 23 and 16%, respectively.
In one of the multicenter studies [19], which lasted 13 weeks, 344 patients with dementia of various etiologies with behavioral disorders, agitation and aggressiveness were divided into 3 groups: patients in group 1 were prescribed risperidone (at a dose of 0.5 to 4 mg/day). days), patients in group 2 were prescribed equivalent doses of haloperidol, patients in group 3 were prescribed placebo. At the end of the study, the mean effective doses of risperidone and haloperidol were found to be 1.1 and 1.2 mg/day, respectively; In a significantly larger number of patients treated with risperidone compared to haloperidol and placebo, there was a reduction in the severity of behavioral and psychotic symptoms (by 30% or more on the BEHAVE-AD scale) and aggression on the CMAI questionnaire), as well as on the Clinical Global Impression (CGI) scale ). Risperidone therapy was well tolerated by patients; there were no significant differences in the appearance of extrapyramidal symptoms in the groups of patients receiving placebo (in 11%) and risperidone (in 15%), whereas in the group of patients treated with haloperidol, they occurred significantly more often (in 22 % of patients).
In a double-blind crossover study [59] involving 120 patients with dementia of various origins (Alzheimer's disease, vascular and mixed dementia), the effectiveness and tolerability of therapy with risperidone (0.5-1.5 mg/day) and haloperidol for 18 weeks was studied. . When taking risperidone compared to haloperidol, patients achieved significantly more pronounced improvement on all scales used to assess the relief of psychotic symptoms and behavior disorders - according to the BEHAVE-AD-K scales (Korean version - BPSD), CGI, Cohen-Mansfield questionnaire - Cohen -Mansfield Agitation Inventory (Korean version - CMAI-K). Risperidone had the additional benefit of reducing aggression and anxiety-phobic disorders. In addition, risperidone was found to have additional benefits in reducing aggression and anxiety and phobias. The risk of antipsychotic-induced extrapyramidal disorders was significantly lower with risperidone use throughout the study.
Only a few studies have examined the effect of risperidone on psychotic and behavioral disorders in patients with dementia, taking into account the drug's effect on cognitive deficits.
In an 8-week open-label study [63], the efficacy of risperidone at a dose of 0.25–2.0 mg/day was assessed in 58 patients with Alzheimer's disease, with a mean age of 75.7 years. 48 (83%) patients completed the study. The mean dose of risperidone at the beginning and end of the study was 0.6 and 1.0 mg/day, respectively.
With a high degree of reliability, a reduction in the level of severity of almost all behavioral and psychotic symptoms (according to the BEHAVE-AD-K scale) was achieved, for example, paranoid, delusional, hallucinatory, affective symptoms decreased, and aggressiveness decreased. No significant changes in cognitive functions were noted (according to the Korean version of the MMSE (Mini-Mental State Examination) [22]. Side effects during treatment with risperidone in most patients were assessed as insignificant.
In an open-label, 8-week study [53], the efficacy of risperidone at varying doses ranging from 0.5 to 2.0 mg/day was assessed in 34 patients with dementia with a mean age of 76 years. At the end of the study, risperidone at a dose of 0.6 mg/day was taken by 18% of patients, 1.0 mg/day by 50%, and more than 1 mg/day by 32%. Assessment of the dynamics of the patients' condition using CGI showed that 82% of patients had significant or very significant improvement. According to the Neuropsychiatric Inventory (NPI) psychotic state questionnaire, the frequency and severity of delusional, hallucinatory symptoms, agitation, aggression and irritability decreased significantly compared to the initial level (p<0.001), but no significant changes in cognitive functions were noted on the MMSE and other scales was. Tolerability of risperidone was assessed as good, and no clinically significant extrapyramidal symptoms, changes in vital signs or body weight were detected.
A study by D. Jeste et al. [32] showed that in elderly patients with schizophrenia complicated by dementia, traditional antipsychotics act less effectively on negative symptoms compared to atypical antipsychotics, including risperidone, while atypical antipsychotics have a better effect on cognitive function.
These data suggest that atypical antipsychotics should be considered as first-line treatment in elderly patients with dementia in combination with psychotic and behavioral disorders. When choosing a therapeutic strategy, one should be guided by the principle of using minimal doses and differentiated treatment in accordance with the spectrum of psychotropic activity of drugs, the characteristics of their effect on neurocognitive deficits and the profile of side effects.
We came across only one domestic study [7] devoted to studying the effectiveness and safety of risperidone in patients with dementia with psychotic and behavioral disorders. However, it did not assess the dynamics of changes in cognitive functions.
The purpose of this work is to assess the clinical effectiveness of low doses of risperidone in the treatment of patients with psychotic disorders and behavioral disorders with dementia of various origins, as well as to assess its effect on cognitive functions in these patients.
Material and methods
The study was conducted in 2010 on the basis of the 6th department of the St. Petersburg City Psychiatric Hospital No. 6. The open-label, prospective, placebo-uncontrolled study included 30 women with various clinical forms of dementia. Their ages ranged from 65 to 89 years (average: 77.6±5.7 years).
The distribution of patients by diagnosis is shown in Table. 1.
| Table 1 |
24 patients had a disability due to mental illness.
Before inclusion in the study, 8 patients received antipsychotics (chlorprothixene, haloperidol, tiapridal, quetiapine).
Inclusion criteria for the study were: a clinical diagnosis of dementia, the absence of concomitant severe or unstable somatic pathology (any condition that directly threatens the patient’s life, malignant neoplasms, acute myocardial infarction, unstable angina, threatening arrhythmias or uncontrolled arterial hypertension, decompensated diabetes mellitus or other endocrinopathies in stages of decompensation, decompensated cardiac, pulmonary, hepatic or renal failure; tuberculosis, HIV, syphilis).
The duration of the study was 4 weeks. Patients included in the study were prescribed rispolux tablets (risperidone) manufactured by Sandoz-Novartis. It was prescribed in a dose of 1 to 2 mg/day in 1 dose, usually at night. During treatment with Rispolux, other antipsychotics were excluded. Only the use of tranquilizers as a hypnotic, antiparkinsonian drugs when extrapyramidal symptoms appeared, and also drugs for the treatment of concomitant somatic diseases were allowed.
The clinical study was carried out in accordance with GCP rules using a specially developed protocol and using unified individual patient records. Psychometric assessment was carried out using the MMSE [48] and CGI-I (Clinical Global Impression Scale - Improvement) scales [30]. It was carried out twice - before the start of therapy and after 4 weeks of taking the drug. Extrapyramidal disorders were assessed using the ESRS (Extrapyramidal Symptom Rating Scale). Before inclusion in the study and after its completion, the patient's weight and waist circumference were measured.
The Statistica 8.0 program was used for data processing.
Results and discussion
Of the 30 patients included in the 4-week study, 29 completed the study. One patient died of sudden onset acute heart failure unrelated to antipsychotic therapy on day 5 of the study.
At the end of the course of therapy, patients receiving different doses of the drug were distributed as follows: 14 (48.2%) patients received a dosage of 1 mg/day, 12 (41.3%) patients received 1.5 mg/day, 2 mg/day — 3 (10.3%). The average daily dose of risperidone was 1.31±0.33 mg.
By the time the study was completed, most patients showed positive dynamics in their mental state. The average score on the CGI-I scale statistically significantly (p<0.01) decreased from 5.41±0.63 (at the beginning of the study) to 3.21±0.56 points (by the end of the study).
By the time the course of therapy was completed, an improvement in mental state was found in 23 (76.6%) patients. During treatment with risperidone, delusional, hallucinatory and behavioral disorders gradually decreased. As a rule, psychotic and behavioral disorders were relieved during the first 2 weeks of treatment, and subsequently the condition did not change significantly. In patients, delirious disorders, confusion, psychomotor agitation, a tendency to aggression were reduced (threats of violence and attempts to attack people around them decreased, aggression at the verbal level), disinhibition and conflict, disturbances of the circadian rhythm (the number of problems with falling asleep, frequent awakenings and shortening of night sleep), disturbance of activity (aimless activity has decreased, inappropriate behavior in the form of excessive sociability with strangers or, conversely, complete detachment from contacts, exposure, storing objects in inappropriate places, etc.). During treatment with Rispolux, a decrease in the manifestations of affective disorders (depressive and anxious mood, tearfulness) was also noted. Reduction of psychotic and behavioral disorders in patients played a key role in restoring cognitive functions and, accordingly, improving social functioning and quality of life. Medical personnel who took part in the therapy and care of patients noted that during treatment with Rispolux, the patients’ behavior and activity were streamlined, the perception of both general information and treatment recommendations improved, the patients became more conscious, which correlates with the data of the study by V.I. . Maksimov [9]. When communicating with others, patients began to feel friendly and benevolent, difficulties in communication significantly decreased, and it became easier to carry out the treatment and rehabilitation process and care for patients.
As for cognitive functions, a statistically significant (p<0.01) improvement was noted on the MMSE scale (Table 2).
| Table 2 |
| ]]> |
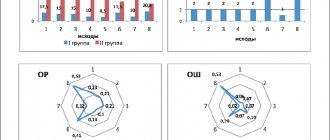
Compared to the initial level (14.97±3.90) by the end of the course of treatment (16.69±3.97), the severity of improvement in these indicators (according to the increase in score) was 1.72±1.60 points, t- criterion - 5.79. The most pronounced statistically significant improvement was observed in such subscales as “orientation in time,” “orientation in place,” and “perception” (Table 3, figure).
| Table3 |
| ]]> |
| Dynamics of main indicators of mental and cognitive status. |
| ]]> |
Indicators on the subscales “concentration and counting” and “speech functions” improved to a somewhat lesser extent.
The existing improvement on the “memory” subscale was statistically insignificant (t-test 1.8; p=0.083). Extrapyramidal disorders assessed by the ESRS were absent throughout the study. Average body weight and waist circumference did not change significantly during the study.
In conclusion, it can be noted that the study showed the high effectiveness of risperidone in the treatment of psychotic and behavioral disorders in patients with dementia. During the first 2 weeks, delirious disorders, confusion, psychomotor agitation, tendency to aggression, disinhibition and conflict were reduced in patients, which undoubtedly influenced the restoration of cognitive functions.
A study of the cognitive status of the studied patients revealed a significant increase in the total score on the MMSE scale. The most pronounced improvement was in the subscales “orientation in time,” “orientation in place,” and “perception.”
Reduction of psychotic (hallucinatory-delusional), behavioral and cognitive disorders in patients contributed to the restoration and improvement of social functioning and quality of life.
The improvement in cognitive functions appeared to be of a secondary nature and was associated with a reduction in psychotic manifestations. This, in our opinion, is evidenced by the fact that the improvement in the “memory” subscale of the MMSE scale did not reach a statistically significant level. From the data obtained in the course of the study, it follows that with adequate therapy with the atypical antipsychotic rispolux (risperidone), cognitive functions in patients with dementia can improve.
In the doses used (1-2 mg/day), Rispolux is well tolerated by patients with dementia with psychotic and behavioral disorders. When assessing extrapyramidal disorders using the ESRS scale, no increase in the incidence of side effects was detected. During the study, there were also no statistically significant changes in the average body weight and waist circumference of the patients.
Indications for use
There are a number of ailments for which Risperidone tablets are particularly effective. The main ones include:
- Schizophrenia (acute or chronic). The active substance makes it possible to carry out treatment at a symptomatic level.
- Psychotic states. Helps eliminate emotional detachment, delusions, poor speech, etc.
- Depression, if accompanied by anxiety.
- Behavioral disorders, including outbursts of anger, severe agitation.
- Dementia if aggressiveness is present.
- Bipolar disorders. Treatment of mania.
- Autism in children and adolescents. The tool allows you to fight auto-aggression.
The drug is also used in the treatment of relapses if a person is diagnosed with chronic schizophrenia and acute psychotic states periodically appear.
Long-term prospects for long-term treatment with risperidone in children with schizophrenia
Risperidone, introduced into practice about 10 years ago, has a unique activity, acting on both D2-dopamine and 5HT2A-serotonin receptors. In relation to α1-adrenergic, H2-histamine and M1-muscarinic receptors, it has less affinity than traditional antipsychotics, however, with its use, a number of side effects may occur, such as nausea, increased appetite, excess weight, galactorrhea, etc. . Currently, risperidone is successfully used in the treatment of acute and chronic forms of schizophrenia in adult patients. The effectiveness of risperidone in the treatment of negative symptoms of schizophrenia and forms resistant to traditional therapy is especially strongly emphasized [3,4,7–11]. An analysis of modern publications shows that risperidone is still little used in domestic child psychiatry. At the same time, foreign psychiatrists use this drug in children to treat hyperactivity, impulsivity, aggression, stereotypic behavior, and obsessive-compulsive disorders. A number of foreign psychiatrists suggest that atypical antipsychotics, in particular risperidone, can play an important role in the treatment of severe autistic disorders [11,17–20]. The relative safety of risperidone (the absence of toxic and mutagenic effects in the experiment, the absence of complications when taking average daily doses by middle-aged people), as well as the noted facts of overcoming therapeutic resistance in the treatment of severe forms of endogenous diseases in adults [4,19] make risperidone attractive for its use in children's practice. In order to study the clinical effectiveness of risperidone in the treatment of intractable, early-onset forms of childhood schizophrenia, children aged 3–12 years (average age 6.4±2.3 years) were treated with risperidone in 1999–2006. under supervision at the Department of Early Childhood at the Mental Health Research Center of the Russian Academy of Medical Sciences. This report is devoted to the follow-up of these results. The criteria for inclusion in the observation group were severe negative and positive mental disorders, disease duration of more than a year, and resistance to previous therapy. Cases with psychoorganic syndrome, mental retardation, convulsive attacks, and severe somatic diseases were not included in the study. Before treatment began, all children were examined by a psychiatrist, neurologist, pediatrician, and psychologist. EEG, ECG, clinical tests of blood, urine, etc. were carried out. In addition, control examinations of the somatic condition of patients, monitoring of biochemical parameters and ECG were regularly prescribed over time. For the use of risperidone in children, informed consent was obtained from parents with the condition of regular visits to the doctor, as well as parents recording the mental and physical state of the child according to a certain scheme. In addition, permission from the ethics committee of the Scientific Center for Mental Health of the Russian Academy of Medical Sciences was obtained for the use of risperidone in children. To assess the effectiveness of risperidone, standardized scales adapted to childhood were used: positive and negative symptoms (PANSS), clinical global impression (CGI). They noted the severity of clinical manifestations, their dynamics, as well as the degree of improvement or deterioration of the condition using a seven-point system [5]. Possible complications and side effects of therapy were recorded using the UKU scale [5,6]. The drug was considered effective if it reduced the total mental state score on the PANSS scale by 20% or more. To avoid possible adverse reactions of risperidone when used in children, we calculated a safe dosage range for the drug, based on theoretical developments of the so-called therapeutic “small dose effect”, known in medicine. The phenomenon of specific cascade responses in the body caused by “small doses” allows one to achieve the same treatment results as when taking a standard macrodose, and at the same time helps to avoid unnecessary complications [7]. Children aged 3–4 years were prescribed 1/20 of the average dose for adults, children 4–5 years old – 1/10 of the dose. Taking into account the rate at which risperidone is eliminated from the body (36 hours or more), in a number of cases, the technique of fractional (every other day) administration of the drug was used. In some cases, the maximum dose of risperidone did not exceed 0.5–0.6 mg/day, which corresponded to the recommendations of leading foreign child psychiatrists [1,11,20,21]. Treatment of patients was not limited to risperidone monotherapy, but in some cases was combined with the use of other antipsychotics - etaprazine, neuleptil and haloperidol in small doses (1/5–1/4 of the average therapeutic dose per day). These drugs were used during the period of exacerbation of psychotic symptoms for a short period of time (1–1.5 weeks). In several cases, the EEG revealed slow-wave peak-like paroxysmal activity, which was clinically expressed by dysphoric outbursts, increased impulsivity and aggressiveness. To mitigate these disorders, sodium valproate (50–150 mg per day) was occasionally added to the main risperidone therapy in small doses. In addition, basic treatment with risperidone was combined with the use of biotics - cerebrolysin, cerebramin, cortexin, biolan, deltaran, etc. The first clinical observation of the effectiveness of risperidone was carried out from 1999 to 2002. The second stage of studying the effectiveness of risperidone was carried out from 2002 to 2006. in 49 children aged 4–12 years (38 boys and 11 girls), also with early onset schizophrenia. The diagnosis of the disease in both groups was qualified according to ICD-10 and DSM-4 as an undifferentiated form of schizophrenia, childhood type. The average age of children in both groups was 5.5±1.2 years, the average duration of the disease was 4.5±1.7 years. When selecting patients for treatment with risperidone, their condition was determined by severe mental disorders within the framework of malignant childhood schizophrenia, or according to ICD 10 - F20.8xx3. The clinical characteristics of the cases included in the observation cohort were determined by catatonic-regressive attacks, accompanied by a suspension of mental development, pronounced autistic manifestations, undulating psychotic symptoms, mutism with the subsequent formation of a specific oligophrenia-like defect. Against this background, repeated exacerbations of the disease were noted in the form of increased affective, catatonic disorders, rudiments of delirium and hallucinations. According to the PANSS scale, the level of violations was determined in the range of 102–165 points (average - 132 points). According to the CGI scale, the severity of the disease was assessed at 6–7 points. From the first two months of treatment, all observed children showed a clear positive effect with a significant reduction in productive and negative symptoms. Impulsive catatonic agitation disappeared from productive symptoms, and the severity of delusional and hallucinatory symptoms decreased. By the end of the first year, the smoothing out of the negative manifestations of the disease was especially noticeable. At the same time, positive symptoms persisted in a number of cases, but were mild and transient in nature. Against this background, seasonal exacerbations of the disease were observed, which, however, did not reach the severity of the manifestations of the primary episodes. Autistic symptoms in patients were noticeably reduced. Their interaction with surrounding children and adults has improved significantly. During communication, eye contact appeared, negativism, manifestations of pseudo-deafness and pseudo-blindness decreased. Social behavior also became more adequate. Emotional contact with loved ones and acquaintances was especially positive. Elements of empathy, a sense of tact, and humor appeared in the children’s behavior, i.e. emotional resonance is adequate to the mood of others. There has been progress in cognitive development - primarily an increase in vocabulary in impressive speech, as well as in some cases expressive speech in the form of individual words and phrases that children used for communicative purposes to express their feelings and exchange impressions with loved ones. Several children's cognitive interests (playing, everyday) expanded. In all cases, an improvement in mood and somatovegetative status was revealed - turgor and skin color normalized, and shine in the eyes and hair appeared. However, the further dynamics of the disease in the cohort of patients turned out to be different, and according to the effectiveness of risperidone therapy, the cohort was divided into two groups. In 17 (35%) patients assigned to the group of malignant schizophrenia, the mental state at follow-up remained relatively severe, and therefore they did not enter general educational institutions on time and were not systematically educated at home. But at the same time, the children were adapted to the microsocial conditions of the family, mastered the basic hygienic skills of self-care, and simple elementary phrasal speech. Some of them were able to learn to read and write on a computer, made attempts to compose simple stories of everyday content, kept a diary, and completed some training programs, although in general cognitive activity remained dissociated, specific thinking disorders persisted (diversity, slippages, cliffs), and the level of lag in intellectual development reached severe mental retardation. In the other 32 patients (65%), who also suffered catatonic-regressive attacks in early childhood, the dynamics during long-term therapy with risperidone turned out to be different. In children, the progression of the disease noticeably changed and became smaller. During therapy with risperidone, the children developed quite developed speech. At the age of 7–8, these children were able to enter correctional schools, where they studied according to a mass program, but in gentle conditions; 6 patients, while undergoing treatment, entered universities. The mental state of these patients still retains mild autistic, affective and catatonic manifestations in speech, motor skills, behavior, as well as individual schizotypal stigmas at the level of emotions, the autonomic nervous system, and individual motor disorders. The condition of 9 children and adolescents is currently determined by neurosis-like and psychopath-like symptoms, episodic, often seasonal, affective fluctuations, against the background of which fragmentary productive symptoms are noted. At the same time, during treatment with risperidone, all these children show a fairly high performance capacity, concentration of attention, thinking, and criticism of their condition. Some of these children developed new interests, hobbies (one for music, another for organizing a business and making money), a tendency to develop and enrich their personality, and expand the range of age-related interests. On the PANSS scale, a year after the start of therapy, there was a decrease in the group average total score from 132 to 84 points (ranging from 90 to 42 points), after 6 years - to 64 points in the subgroup with an unfavorable course and to 48 points in the subgroup with a favorable course . Figure 1 shows the dynamics of the decrease in the average total score of the PANSS scale, reflecting a decrease in the severity of the mental state of patients during treatment (p<0.005). The average percentage improvement for the group during follow-up from 1999 to 2006 was 52%. The severity of the condition on the CGI scale decreased to 4 points. According to the UKU scale, the severity of complications did not exceed 1–2 points. During treatment with risperidone, some of the patients, at the initiative of their parents, took a long (up to 3–6 months) break from taking the drug. After a break of 4–5 months, they experienced a return of previous psychotic symptoms to a lesser extent than before treatment, however, the patients lost their previous working capacity and activity, and interrupted the educational process at school and college. Resumption of risperidone treatment again improved their mental status. These facts led to the conclusion that interrupting treatment with risperidone was inappropriate. But due to the need for long-term use of the drug, certain measures were taken to prevent therapeutic addiction directly to risperidone. For this purpose, breaks were taken in treatment for 1–2 summer months or an intermittent course of treatment was prescribed in the form of 0.1–0.2 mg 2–3 times a week, which was pharmacokinetically justified, because up to 75% of the drug and its derivatives remained in circulation in the patient’s blood for 2–3 days. In conclusion, it should be added that during long-term therapy with risperidone, no serious side effects clearly associated with taking the drug were recorded. According to the UKU scale, only minor autonomic abnormalities were noted, mainly at the beginning of therapy (low-grade fever, sleep disturbances, headaches, discomfort in the epigastric region); in isolated cases, allergic manifestations in the form of skin rash and slight weight gain were noted, which disappeared on their own and did not lead to discontinuation of the drug. Thus, summing up the results of long-term therapy with risperidone, we can conclude that treatment with small doses of the drug is effective and safe in childhood. It was after a one-year period of treatment that improvements in cognitive functions, attention, performance, thinking, speech, and social behavior became noticeable. Treatment with risperidone, apparently, not only alleviated the current condition of patients, but also generally influenced the pathogenesis of the disease. This hypothesis is confirmed by the fact that to date, in the vast majority of patients, the disease has acquired a regressive course, regardless of their initial condition. Since the reduction of psychopathological manifestations was achieved in the process of complex treatment, it is advisable to recommend a combination of risperidone with small doses of other antipsychotics and courses of biotics. Observation shows that a gentle treatment regimen and an individual approach to the choice of therapeutic dosages, refusal to force maximum daily dosages allows the use of risperidone for a long time without the risk of complications for the physical health of children.
Literature 1. Arena D., Rosenbaum D. Pharmacotherapy of mental disorders. Per. from English – M.: BINOM, 2004. – P. 61–63. 2. Beznos S.A., Shaposhnikov N.N., Ryazanova E.A. Experience in using the drug “rispolept” based on materials from the children’s department of the Psychiatric Hospital of Krasnodar / Materials of the Second Scientific and Practical Conference of Psychiatrists and Narcologists of the Southern Federal District, June 21–23, 2006, Rostov-on-Don. – pp. 29–32. 3. Vovin R.Ya., Mazo G.E., Ivanov M.V., Kosterin D.N. The use of rispolept to relieve exacerbations of schizophrenia // Psychiatry and psychopharmacotherapy. – 2000. – App. No. 2. – pp. 6–8. 4. Kalinin V.V. // Social and clinical psychiatry. – 1999, No. 1. – P. 97–105. 5. Kozlovskaya G.V., Kalinina M.A., Goryunova A.V., Proselkova M.E. Experience of using rispolept in the treatment of early childhood autism and schizophrenia in children // Psychiatry and psychopharmacotherapy. – 2000. – App. No. 2. – pp. 10–12. 6. Kozlovskaya G.V., Kalinina M.A. The effectiveness of rispolept in children in the prolonged (for 2 years) treatment of schizophrenia and early childhood autism // Psychiatry and psychopharmacotherapy. – 2003. – App. No. 1. – pp. 10–13. 7. Kozlovskaya G.V. Kalinina M.A. Goryunova et al. Psychopharmacology in micropsychiatry // Psychiatry and psychopharmacotherapy. – 2006, No. 5, vol. 7. – P. 256–259. 8. Kolyutskaya E.V., Dorozhenok I.Yu., Ilyina N.A. // Social and clinical psychiatry. – 1998, No. 4. – P. 88–91. 9. D.N. Kosterin, G.E. Mazo, M.V. Ivanov // Social and clinical psychiatry. – 2000, No. 1. – P. 46–47. 10. Mosolov S.N., Kalinin V.V., Eremin A.V. et al. Comparative randomized study of the effectiveness and tolerance of risperidone and haloperidol in the relief of acute conditions in patients with schizophrenia and schizoaffective psychosis // Psychiatry and psychopharmacotherapy. – 2000. – App. No. 2. – pp. 3–6. 11. Shinaev N.N., Akzhiginov R.G., Volkova N.P. The use of the atypical antipsychotic rispolept in the clinic of borderline mental disorders // Psychiatry and psychopharmacotherapy. – 2000. – App. No. 2. – pp. 8–10. 12. Guide to Clinical Child and Adolescent Psychiatry: Trans. from English / Ed. K.S. Robson. – M.: Medicine, 1999. – P. 227–255. 13. Armenteros JL, Whitaker AH, Welikson M., et al., Risperidon in ado-lescents with schizophrenia: an open pilot study // J Am Acad Child Adolesc Psy-chiatry 36: 5, 694–700, May, 1997. 14. Carlsson A., Waters N., Carlsson ML, Neurotransmitter interactions in schizophrenia–therapeutic implications // Biol. Psychiatry. – 1999; 46:1388–1395. 15. Crismon M. L, Dorson PG Schizophrenia in: Dipiro JT, Talbert RL, and Yee GC eds. Pharmacotherapy: A Pathophysiologic Approach. New York, NY: McGraw–Hil/Appleton and Lange; 1999. 16. Falkai P, Wobrock T, et al. Guidelines for biological treatment of schizophrenia. Part 1 // World J Biological Psychiatry 6(3), 132–144, 2005. 17. Findling RL, Maxwell K., Wiznizer M. An open clinical trial of risperi-don monotherapy in young children with autistic disorders // Psychopharmacol Bull 33: 1, 155–9, 1997. 18. Kapur S., Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2receptor occupancy of clozapine, risperidon and olanzapine in schizophrenia // Am J Psychiatry 158: 286–293, 1999. 19. Kerwin RW Role of atypical antipsychotic in schizophrenia // Scizophr Bull: 25; 281–282, 2001. 20. Posey DJ, Walsh KH, Wilson GA, et al. Risperidon in the treatment of two very young children with autism // J Child Adolesc Psychopharmacol, 1999, 9, 273–276. 21. Stahl SM Psychopharmacology of Antipsychotics, 1999, M Dunits, London Reprinted, 2000, USA. – 77, 109–119. 22. Tasman A., Kay J., Lieberman JA Psychiatry, Second Ed, v. 1, Jwilly a Sons, LTD, 2004: 770–773. The article was published in the journal “Issues of Mental Health of Children and Adolescents”. – 2006 (6), No. 2, pp. 56–62.
Contraindications and restrictions
The use of Risperidone is completely prohibited in the following cases:
- if the patient has paroxysmal epilepsy;
- with Parkinson's disease;
- during breastfeeding;
- in the presence of hypersensitivity to the drug itself.
As for pregnancy, the use of the medicine during this period is not recommended. However, if its use causes less harm to the fetus than refusing therapy with this substance, the attending physician has the right to prescribe such a drug. However, the treatment itself must be supervised by a specialist.
There are also a number of diseases, in the presence of which the pills should be taken carefully with strict control of the dose and the body’s reaction. Their list includes the following:
- diseases of the cardiovascular system;
- cerebrovascular accident;
- dehydration.
In addition, such an active substance is not recommended to be combined with other drugs that affect the central nervous system. If such therapy turns out to be mandatory, it is necessary to correctly determine the dose of each of the drugs used.
Risperidone Organica, 4 mg, film-coated tablets, 20 pcs.
Transition from therapy with other antipsychotic drugs.
In schizophrenia, at the beginning of treatment with Risperidone Organica, it is recommended to gradually withdraw previous therapy if clinically justified. If patients are transferred from depot therapy with antipsychotic drugs, it is recommended to start taking Risperidone Organic instead of the next scheduled injection. The need for continued therapy with antiparkinsonian drugs should be periodically assessed. Due to the α-adrenergic blocking effect of Risperidone Organic, orthostatic hypotension may occur, especially during the initial dose selection period. If blood pressure decreases, dose reduction should be considered. In patients with cardiovascular diseases, as well as in cases of dehydration, hypovolemia or cerebrovascular disorders, the dose should be increased gradually, according to recommendations. The occurrence of extrapyramidal symptoms is a risk factor for the development of tardive dyskinesia. If signs and symptoms of tardive dyskinesia occur, discontinuation of all antipsychotic medications should be considered.
Antipsychotic medications, including Risperidone Organica, should be prescribed with caution to patients with Parkinson's disease or dementia with Lewy bodies. Both groups of patients have increased sensitivity to antipsychotic drugs (including dullness of pain sensitivity, confusion, postural instability with frequent falls and extrapyramidal symptoms), as well as an increased risk of neuroleptic malignant syndrome, characterized by hyperthermia, muscle rigidity, instability of autonomic functions, disturbances of consciousness and increased CPK activity (myoglobinuria (rhabdomyolysis) and acute renal failure may also occur), in these cases it is necessary to discontinue all antipsychotic drugs, including Risperidone Organica. When discontinuing carbamazepine and other liver enzyme inducers, the dose of Risperidone Organic should be reduced.
In elderly patients with dementia, increased mortality is observed when treated with atypical antipsychotics, including risperidone, compared with placebo. When using Risperidone in this population, the incidence of death was 4% for patients taking Risperidone, compared with 3.1% for placebo. The mean age of patients who died was 86 years (range, 67–100 years). In elderly patients with dementia taking oral forms of risperidone, increased mortality was observed in patients taking furosemide and risperidone (7.3%, mean age 89 years, range 75–97 years) compared with the group taking risperidone alone (4.1 %, mean age 84 years, range 75–96 years) and the furosemide only group (3.1%, mean age 80 years, range 67–90 years). No pathophysiological mechanisms have been established to explain this observation. However, special care should be taken when prescribing the drug in such cases. No increase in mortality was found in patients concomitantly taking other diuretics with Risperidone. Regardless of treatment, dehydration is a common risk factor for mortality and should be carefully monitored in older patients with dementia.
Hyperglycemia, diabetes mellitus, or exacerbation of existing diabetes mellitus were observed during treatment with the drug. Establishing a relationship between the use of atypical antipsychotic drugs and disorders of glucose metabolism is complicated by the increased risk of developing diabetes mellitus in patients with schizophrenia and the prevalence of diabetes mellitus in the general population. Given these factors, the relationship between the use of atypical antipsychotic drugs and the development of adverse effects associated with hyperglycemia is not fully established. All patients should be clinically monitored for symptoms of hyperglycemia and diabetes mellitus.
A significant increase in body weight was observed during treatment with Risperidone. It is necessary to monitor the body weight of patients during therapy with Risperidone. Caution should be exercised when prescribing Risperidone Organic to patients with a history of cardiac arrhythmias, patients with congenital prolongation of the QT interval, and when used together with drugs that increase the QT interval. The ability of typical antipsychotics to lower the seizure threshold is known. Risperidone Organica should be prescribed with caution to patients with epilepsy. Therapy with the powerful D2 receptor antagonist Risperidone Organica can lead to the development of pituitary tumors, hyperprolactinemia and galactorrhea.
The use of antipsychotic drugs can cause dysphagia and impaired esophageal motility. Risperidone Organic and other antipsychotics should be used with caution in patients with Alzheimer's dementia who are at increased risk of aspiration pneumonia. Patients should be advised to refrain from overeating due to the possibility of weight gain.
During treatment you must refrain from drinking alcohol.
Gradual withdrawal of the drug is recommended, because after abrupt cessation of treatment with high doses of antipsychotics, withdrawal syndrome may develop (vomiting, nausea, increased sweating, insomnia).
Side effects
Although the drug is considered safe, in some cases certain side effects may occur. These include:
- Drowsiness, fatigue, decreased concentration, tremors or seizures.
- Abdominal pain, nausea, vomiting, constipation, sudden changes in body weight - decrease or increase.
- Pressure surges.
- Decreased libido and sexual dysfunction.
- Autoimmune disorders.
- Skin rash, dry skin, itching.
- Allergic rhinitis.
- Deterioration of vision.
Most often, such symptoms are temporary and disappear after finishing the course of taking the pills. However, if any side effects are detected, you must inform your doctor about it.