Forsteo solution for subcutaneous injection 250 µg/ml 2.4 ml (Eli Lilly)
Teriparatide is a recombinant human parathyroid hormone produced using a strain of Escherichia coli (using DNA recombination technology). Endogenous parathyroid hormone (PTH), which is a sequence of 84 amino acid residues, is the main regulator of calcium and phosphorus metabolism in the bones and kidneys. Teriparatide (recombinant human PTH (1-34)) is an active fragment of endogenous human PTH. The physiological effect of PTH is to stimulate bone formation through a direct effect on osteoblasts. PTH indirectly increases intestinal absorption and tubular reabsorption of calcium, as well as renal excretion of phosphate. Pharmacodynamic properties The biological effect of PTH is due to binding to specific PTH receptors on the surface of cells. Teriparatide binds to the same receptors and has the same effects on bones and kidneys as PTH. Single daily administration of teriparatide stimulates the formation of new bone tissue on the trabecular and cortical (periosteal and/or endosteal) surfaces of bones with preferential stimulation of osteoblast activity relative to osteoclast activity. This is confirmed by an increase in the levels of markers of bone tissue formation in the blood serum: bone-specific alkaline phosphatase and carboxy-terminal propeptide of type I procollagen (PICP). The increase in the content of bone tissue formation markers is accompanied by a secondary increase in the level of bone resorption markers in urine: N-telopeptide (NTX) and deoxypyridinoline (DPD), which reflects the physiological interaction of the processes of bone tissue formation and resorption in skeletal remodeling. 2 hours after administration of teriparatide, a short-term increase in serum calcium concentration is observed, which reaches maximum values after 4-6 hours and returns to baseline values within 16-24 hours. In addition, transient phosphaturia and a slight short-term decrease in serum phosphorus levels may occur. Clinical effectiveness. Postmenopausal osteoporosis. The main clinical trial of teriparatide included 1637 patients with postmenopausal osteoporosis, with a mean age of 69.5 years. At the start of the study, 90% of patients had suffered 1 or more vertebral fractures and the mean vertebral bone mineral density (BMD) was equivalent to a T-score of -2.6. All patients took 1000 mg of calcium daily and. at least 400 IU vitamin D. Results of teriparatide therapy for up to 24 months (median duration of therapy was 19 months) indicate a statistically significant reduction in the incidence of fractures. The incidence of new vertebral fractures (>1 fracture, as measured by baseline and end-line radiography) in the teriparatide group and placebo group was 5.0% and 14.3%, respectively (p2 fractures, as determined by baseline and end-line radiography). study) in the teriparatide group and placebo group were 1.1% and 4.9%, respectively (p
Forsteo solution for subcutaneous administration 250 mcg/ml 2.4 ml syringe pen 1 pc. in Moscow
PC,
in the thigh or abdomen area. The recommended dose of teriparatide is 20 mcg, administered once a day. The maximum duration of treatment with teriparatide is 24 months.
The effectiveness and safety of teriparatide during therapy for more than 2 years have not been studied; therefore, teriparatide therapy for more than 24 months during the patient's lifetime is not recommended.
Supplementation with calcium and vitamin D is recommended if dietary intake is insufficient. The dosage does not depend on the patient's age. The patient must be trained in the technique of administering the drug (see “Guide to using a syringe pen”).
Instructions for using a syringe pen
The drug Forsteo® is a solution in a syringe pen intended for individual use. A new sterile needle is required for each injection. Each package of Forsteo® contains a Patient Guide that describes in detail the rules for handling the syringe pen.
Injection needles are not included. The pen can be used with insulin pen needles (Becton Dickinson)
. The drug should be administered immediately after the syringe pen is removed from the refrigerator. After each injection, the syringe pen should be placed in the refrigerator.
Colter™ teriparatide injection. Application Guide
Before using your new Kolter™ pen, please read this Instructions for Use (on both sides) in its entirety.
When using the Kolter™ syringe pen, you must strictly follow the instructions.
You cannot give your syringe pen and needles to anyone, because... this may lead to transmission of infection.
The Kolter™ syringe pen contains a dose of the drug for 28 days.
Details* Kolter™ syringe pens
Trigger button (black), stem (yellow) with red stripe, body (blue), medicine cartridge, cap (white), paper tongue, inner protective cap, outer protective cap.
* (Needles not included. Becton, Dickinson and Company (BD)
29–31 caliber).
You must wash your hands before each injection. Prepare the injection site as directed by your healthcare provider.
1. Remove the white cap.
2. Attach a new needle:
- tear off the paper tongue;
— insert the needle directly into the cartridge with the drug;
- screw the needle tightly;
— remove the outer protective cap and set it aside without throwing it away.
3. Set the dose:
— pull out the black start button as far as it will go (if you can’t pull out the black start button, you should refer to the Troubleshooting
, problem D, on the reverse side);
— make sure that the red stripe is visible;
— remove the inner protective cap and throw it away.
4. Administer dose:
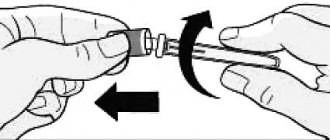
— gently pull back the skin on the thigh or abdomen and insert the needle subcutaneously;
— press the black start button all the way. While holding, slowly count to 5. Then remove the needle.
5. Check the dose after injection:
— after removing the needle, make sure that the black trigger button is pressed in all the way. If the yellow rod is not visible, the injection was performed correctly. The patient should not see the yellow rod. If the yellow rod is visible after the injection, do not re-inject on the same day. The patient must return the Colter™ pen to its original position (see Troubleshooting
, problem A, on the reverse side).
6. Remove the needle:
— put the outer protective cap on the needle;
— completely unscrew the needle by turning the outer protective cap 3–5 times;
- remove the needle and dispose of it in accordance with the recommendations of the attending physician;
— put the white cap back on and place the Kolter™ syringe pen in the refrigerator immediately after use.
Colter™ syringe pen injection of teriparatide, troubleshooting
A. The yellow rod is still visible even though the patient has pressed the black trigger button. How can he return the Colter™ syringe pen to its original position?
To return the Colter™ pen to its original position, you must follow the instructions below.
1) If the patient has already given an injection, do not re-inject on the same day.
2) Remove the needle.
3) Attach a new needle, remove the outer protective cap and set it aside without throwing it away.
4) Pull the black start button as far as it will go. Make sure the red stripe is visible.
5) Remove and discard the inner protective cap.
6) Point the needle into an empty container. Press the black start button as far as it will go. While holding, slowly count to 5. The patient may see fluid or droplets of fluid leaking out. At the end of the procedure, the black start button must be pressed all the way.
7) If the yellow rod is still visible, you should contact the Company or your healthcare provider.
 Place the outer protective cap on the needle. Unscrew the needle completely by turning the outer protective cap 3–5 times. Remove and discard the needle as directed by your healthcare provider. Replace the white cap and place the Colter™ pen in the refrigerator.
Place the outer protective cap on the needle. Unscrew the needle completely by turning the outer protective cap 3–5 times. Remove and discard the needle as directed by your healthcare provider. Replace the white cap and place the Colter™ pen in the refrigerator.
This problem can be prevented by using a new needle for each injection and pressing the black trigger button all the way in and slowly counting to 5.
B. How can I make sure that the Colter™ syringe pen works?
The Kolter™ syringe pen is designed specifically to administer a full dose of the drug with each use in accordance with the Directions for Use
. When the black trigger button is pressed in all the way, the patient can be confident that the full dose of medication has been administered using the Colter™ pen.
To ensure that your pen works properly, remember to use a new needle each time.
Q. An air bubble is visible in the syringe pen.
A small air bubble will not affect the dose of the drug and will not harm the patient. The patient can continue dosing as usual.
D. The patient cannot remove the needle.
1) Place the outer protective cap on the needle.
2) Use the outer protective cap to unscrew the needle.
3) Unscrew the needle completely by turning the outer protective cap 3–5 times.
4) If the needle still cannot be removed, contact someone for help.
D. What should I do if the patient cannot press the black trigger button?
Use a new Kolter™ syringe pen to administer a dose that corresponds to the recommendations of your physician.
This indicates that all possible amounts of the drug have been used, even if there is still some left in the drug cartridge.
Care and storage
Caring for the Kolter™ syringe pen.
Wipe with a damp cloth from the outside; Do not place the Colter™ syringe pen in water, do not wash or clean it with any liquids.
Storing the Kolter™ syringe pen.
Place the Kolter™ syringe pen in the refrigerator immediately after use; read the storage conditions in the instructions for use of the medicinal product and follow them; do not store the Kolter™ syringe pen with a needle attached, as this may lead to the formation of air bubbles in the medication cartridge; store the Kolter™ syringe pen with the white cap on; never store the Kolter™ syringe pen in the freezer; If the drug has been frozen, discard the device and use a new Colter™ pen. If the patient forgets to place the Colter™ pen in the refrigerator, the pen should not be thrown away. Place the pen in the refrigerator and contact the manufacturer.
Disposal of pen needles and Kolter™ pen device
— before disposing of the Kolter™ syringe pen, make sure that the needle of the syringe pen is removed;
— dispose of the Kolter™ syringe pen and used needles in accordance with the rules recommended by the attending physician, local and departmental requirements;
— dispose of the device within 28 days after first use.
Forsteo
The parathyroid hormone analogue, which is a sequence of 84 amino acid residues, is the main regulator of Ca2+ and phosphorus metabolism in bone tissue and kidneys. The active substance of the drug is an active fragment of endogenous human parathyroid hormone. The physiological effect of parathyroid hormone is to stimulate bone formation through a direct effect on osteoblasts. Indirectly increases intestinal absorption and tubular reabsorption of Ca2+, as well as phosphate excretion by the kidneys.
The biological effect of parathyroid hormone is carried out by binding to specific receptors on the surface of cells. The active substance of the drug binds to the same receptors and has the same effect on bone tissue and kidneys as parathyroid hormone. Daily single administration of the drug stimulates the formation of new bone tissue on the trabecular and cortical (periosteal and/or endosteal) surfaces of bones with preferential stimulation of osteoblast activity relative to osteoclast activity. This is confirmed by an increase in the content of markers of bone tissue formation in the blood serum: bone-specific ALP and procollagen-1 carboxy-terminal propeptide. An increase in the content of bone tissue formation markers is accompanied by a secondary increase in the concentration of bone resorption markers in urine: N-telopeptide and deoxypyridinoline, which reflects the physiological interaction of the processes of bone tissue formation and resorption in skeletal remodeling. 2 hours after administration of the drug, a short-term increase in the concentration of serum Ca2+ is observed, which reaches maximum values after 4-6 hours and returns to the initial level within 16-24 hours. In addition, transient phosphaturia and a slight short-term decrease in the concentration of phosphorus in the blood serum may be observed. .
During treatment with the drug, bone mineral density (BMD) of the whole body increases by 5-10% (including the lumbar spine, femoral neck and femur).
Mineralization processes occur without signs of toxic effects on bone tissue cells, and the bone tissue formed under the influence of the drug has a normal structure (without the formation of reticulofibrous bone tissue and bone marrow fibrosis).
The use of the drug reduces the risk of developing fractures regardless of age, the initial level of bone metabolism or BMD (the relative reduction in the risk of new fractures is 65%).
Forsteo®
| Mechanism of action |
Teriparatide is a recombinant human parathyroid hormone produced using a strain of Escherichia coli (using DNA recombination technology).
Endogenous parathyroid hormone (PTH), which is a sequence of 84 amino acid residues, is the main regulator of calcium and phosphorus metabolism in the bones and kidneys. Teriparatide (recombinant human PTH (1-34)) is an active fragment of endogenous human PTH. The physiological effect of PTH is to stimulate bone formation through a direct effect on osteoblasts. PTH indirectly increases intestinal absorption and tubular reabsorption of calcium, as well as renal excretion of phosphate. Pharmacodynamic properties The biological effect of PTH is due to binding to specific PTH receptors on the surface of cells. Teriparatide binds to the same receptors and has the same effects on bones and kidneys as PTH.
Single daily administration of teriparatide stimulates the formation of new bone tissue on the trabecular and cortical (periosteal and/or endosteal) surfaces of bones with preferential stimulation of osteoblast activity relative to osteoclast activity. This is confirmed by an increase in the levels of markers of bone tissue formation in the blood serum: bone-specific alkaline phosphatase and carboxy-terminal propeptide of type I procollagen (PICP). The increase in the content of bone tissue formation markers is accompanied by a secondary increase in the level of bone resorption markers in urine: N-telopeptide (NTX) and deoxypyridinoline (DPD), which reflects the physiological interaction of the processes of bone tissue formation and resorption in skeletal remodeling. 2 hours after administration of teriparatide, a short-term increase in serum calcium concentration is observed, which reaches maximum values after 4-6 hours and returns to baseline values within 16-24 hours. In addition, transient phosphaturia and a slight short-term decrease in serum phosphorus levels may occur.
Clinical effectiveness
Postmenopausal osteoporosis
The main clinical trial of teriparatide included 1637 patients with postmenopausal osteoporosis, with a mean age of 69.5 years.
At the start of the study, 90% of patients had suffered 1 or more vertebral fractures and the mean vertebral bone mineral density (BMD) was equivalent to a T-score of -2.6. All patients took 1000 mg of calcium daily and. at least 400 IU vitamin D.
Results of teriparatide therapy for up to 24 months (mean duration of therapy was 19 months) indicate a statistically significant reduction in the incidence of fractures. The incidence of new vertebral fractures (>1 fracture, as measured by radiography at the beginning and end of the study) in the teriparatide group and in the placebo group was 5.0% and 14.3%, respectively (p < 0.001 compared with the placebo group, a relative decrease risk - 65%).
The incidence of multiple vertebral fractures (>2 fractures, as determined by radiography at the beginning and end of the study) in the teriparatide group and in the placebo group was 1.1% and 4.9%, respectively (p < 0.001 compared with the placebo group, relative risk reduction - 77%).
The incidence of nonvertebral low-energy fractures (minimal trauma fractures) in the teriparatide group and placebo group was 2.6% and 5.5%, respectively (p < 0.025 compared with placebo group, relative risk reduction 53%).
The incidence of major nonvertebral low-energy fractures (femur, radius, humerus, ribs, pelvis) in the teriparatide group and placebo group was 1.5% and 3.9%, respectively (p < 0.025 compared with the placebo group, decreased relative risk - 62%).
After 19 months of treatment (median duration of therapy), there was an increase in BMD at the lumbar spine and proximal femur compared to placebo by 9% and 4%, respectively (p < 0.001). Post-therapy follow-up: After completion of teriparatide therapy, 1262 women with postmenopausal osteoporosis from the main study were included in a follow-up study. The primary objective of the study was to collect data on the safety of teriparatide. During this observation period, other osteoporosis therapy was permitted and additional evaluation of vertebral fractures was performed. At an average of 18 months after discontinuation of teriparatide therapy, the number of patients with at least one new vertebral fracture in the teriparatide-experienced group was 41% lower than in the placebo group (p=0.004).
| In an open-label study, 503 patients with postmenopausal severe osteoporosis and fragility fractures (minimal trauma fractures) within the previous three years (83% had previously received treatment for osteoporosis) received teriparatide for 24 months. After 24 months, BMD at the lumbar spine, proximal femur, and femoral neck increased from baseline by an average of 10.5%, 2.6%, and 3.9%, respectively. From 18 to 24 months, BMD at the lumbar spine, proximal femur, and femoral neck increased by 1.4%, 1.2%, and 1.6%, respectively. Osteoporosis in men |
In a clinical study of men with osteoporosis due to hypogonadism (defined by low morning free testosterone or elevated follicle-stimulating hormone or luteinizing hormone concentrations) or
| 437 patients with idiopathic osteoporosis took part, with an average age of 58.7 years. At the start of the study, the BMD of the vertebrae and femoral neck according to the T-criterion ranged from -2.2 to -2.1, respectively. At the start of the study, 35% of patients had a history of vertebral fractures, and 59% of patients had fractures of other locations. All patients took 1000 mg calcium and at least 400 IU vitamin D daily. Significant increases in lumbar spine bone mineral density were noted after 3 months. After 12 months of therapy, BMD at the lumbar spine and proximal femur increased by 5% and 1%, respectively, compared with placebo. |
Osteoporosis with long-term therapy
| glucocorticosteroids The effectiveness of teriparatide in osteoporosis caused by long-term treatment with glucocorticosteroids was proven in an 18-month randomized, double-blind clinical trial with an active comparator (alendronate 10 mg/day; 428 patients, mean age 57 years). At the start of the study, 28% of patients had 1 or more vertebral fractures. All patients took 1000 mg of calcium and 800 IU of vitamin D daily. The study included 277 postmenopausal women, 67 premenopausal women and 83 men. After 18 months of therapy, BMD of the lumbar spine increased by 7.2% (by 3.4% in the alendronate group, p < 0.001), BMD of the proximal femur increased by 3.6% (by 2.2% in the alendronate group, p<0.01), femoral neck BMD increased by 3.7% (by 2.1% in the alendronate group, p<0.05). |
In patients taking teriparatide,
| study period from 18 months to 24 months of therapy, BMD of the lumbar spine, proximal femur, and femoral neck increased by an additional 1.7%, 0.9%, and 0.4%, respectively. In the teriparatide group, after 36 months of therapy, new vertebral fractures were detected in 1.7% of patients (7.7% in the alendronate group, p = 0.01), new non-vertebral fractures were detected in 7.5% of patients (7.0% in the alendronate group, p =0.84). In premenopausal women after 18 months of therapy, the increase in BMD was significantly higher in the teriparatide group compared with alendronate: lumbar spine BMD increased by 4.2% (-1.9% in the alendronate group, p < 0.001), proximal BMD femur increased by 3.8% (0.9% in the alendronate group, p = 0.005). |
Mineralization processes occur without signs of toxic effects on bone tissue cells, and bone tissue formed under the influence of teriparatide has a normal structure (without the formation of reticulofibrous bone tissue and bone marrow fibrosis). Teriparatide reduces the risk of fractures regardless of age, baseline bone turnover, or BMD (relative risk reduction for new fractures is 65%).