Pharmacological properties
Pharmacological. Iron saccharate is an antianemic agent containing ferric iron. Iron sucrose is relatively stable and releases little iron as free ions.
Ferric iron is an essential component of the human diet, necessary for the synthesis of hemoglobin, myoglobin, and respiratory enzymes. Typically, during the day, a person loses from 1 to 2 mg of iron through feces and urine, which accumulates with food. With a balanced diet of iron, iron deficiency does not develop. With increased loss of iron or with pathologies that are accompanied by impaired absorption in the upper parts of the small intestine, iron deficiency develops, which may appear in the form of iron deficiency anemia. Menstruation in women, accompanied by large blood losses, often contributes to the development of iron deficiency anemia. The use of the drug “Iron saccharate - iron wine” helps reduce iron deficiency in the body in case of iron deficiency anemia.
Pharmacokinetics. After taking the drug, absorption of the iron complex occurs in the duodenum and upper parts of the small intestine. Its absorption is not reduced as a result of interaction with food components. Once in the systemic circulation, iron from the blood serum passes into tissues capable of depositing it. In particular, it is deposited in the liver in the form of a complex with ferritin. Later, in the bone marrow it is included in hemoglobin. In the composition of hemoglobin, iron is in divalent form, but it is trivalent iron that stimulates the formation of globin, which helps to increase blood hemoglobin.
The mechanism of absorption of ferric iron is active, depends on the level of iron deficiency in the body and is carried out in such a way that iron is not absorbed in the small intestine in excess of the physiological norm.
Iron is excreted from the body in feces and urine, mainly in the form of hemoglobin metabolic products.
Directions for use and doses
For adults, as well as pregnant women and children over 12 years of age, the drug “Iron sugar - iron wine” is prescribed orally, 1 tablespoon in 0.5 glasses of water three times a day, after meals. It is advisable to suck the solution through a straw to avoid darkening of the teeth, and after taking the drug, it is advisable to rinse your mouth. Periodically, once every few weeks, a blood test is done and the hemoglobin level is determined. When the hemoglobin level normalizes, the drug is stopped.
For children aged 1 to 12 years, the drug is prescribed at the rate of 3 mg of iron per 1 kg of body weight per day, which corresponds to 1 ml of the drug per 1 kg of body weight per day. The calculated daily dose is divided into three doses. The duration of taking the drug is determined by the period of normalization of hemoglobin in the blood.
The Evolution of Intravenous Iron Preparations
The article describes four types of parenteral iron supplements, including intravenous ones. The indications for the use of intravenous iron preparations, the structural features of iron carboxymaltose, the effectiveness and safety of its use in various diseases, proven based on a meta-analysis of randomized clinical trials, are considered.
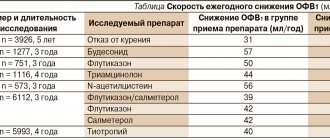
Table 1. Acute toxicity of some iron preparations
Table 2. Some modern iron preparations for intravenous administration
Drawing. Schematic structure of the iron carboxymaltose molecule
In November 2000, the National Anemia Action Council (NAAC) was created in the United States. The council includes 30 leading experts in the diagnosis and treatment of anemia in the fields of hematology, nephrology, oncology, cardiology, rheumatology, gastroenterology, infectious diseases, surgery, geriatrics, and pediatrics. Experts initiated the creation and publication in 2004 of the monograph “Anemia - a hidden epidemic.” This book was translated into Russian and published in Russia in large quantities [1]. In the publication, the authors note several fundamental positions regarding anemia.
First, anemia often goes unrecognized and therefore goes untreated.
Secondly, anemia accompanies many chronic diseases and conditions.
Thirdly, if anemia is not treated, serious consequences are possible - a more severe course of a chronic disease, a decrease in the patient’s quality and life expectancy.
Fourthly, anemia can be treated using modern methods.
Currently, the following methods are used to treat anemia:
- transfusion of red blood cells. Advantages of the method: widespread implementation in clinical practice, immediate effect, relatively low cost. Disadvantages: possibility of transmission of infectious agents, suppression of recipient hematopoiesis, post-transfusion complications, need to determine blood type and Rh factor, shortage of donor personnel [2, 3];
- use of hemoglobin-associated oxygen carrier (Hemopure). Pros: cheap raw materials (bovine red blood cells) for the preparation of the drug, no need to determine the blood type and Rh factor, quick effect, stimulation of the formation of one’s own red blood cells, long shelf life (three years). Disadvantages: introduction of xenogeneic material, little experience in clinical use, lack of data on use in children, adolescents, pregnant and lactating women [4];
- use of erythropoietin (EPO). Pros: the ability to use the drug at any time (if available) and no need for red blood cell transfusion. Disadvantages: high cost of the drug and course of treatment, delayed effect (no earlier than one to two weeks), the need for combination with iron supplements, not always effective use [5];
- use of oral iron supplements. Pros: the most accessible and cheapest method. Cons: not always effective, slow onset of effect [6];
- use of intravenous iron supplements. Pros: availability of drugs, relatively low cost of treatment, complete absence of risk of transmission of infections and suppression of the patient's hematopoiesis, rapid delivery of iron to the bone marrow, possibility of use in combination with EPO. Cons: delayed effect [7].
Features of treatment with intravenous iron preparations were formulated by IC Macdougall [8]:
- this treatment is most guaranteed to deliver iron to the bone marrow;
- patients receiving intravenous iron supplements require careful clinical monitoring;
- a short-term appearance of free iron may be induced;
- due to high toxicity, some drugs are used only in low doses (iron gluconate);
- complications are possible: iron overload, anaphylactoid reactions (caused by dextran), long-term toxicity, increased risk of infections, etc.
Properties and features of parenteral iron preparations
There are four groups of parenteral iron preparations, taking into account kinetic (labile, stable) and thermodynamic (weak, strong) properties. Drugs of these groups differ in the stability of the complex, molecular weight, toxicity, histotoxicity, pharmacokinetics and the presence of adverse events.
Type 1 complexes:
iron dextran and iron dextrin. Both complexes are stable, with a molecular weight of more than 100 kDa. Iron dextran complexes for intravenous administration are also known: low molecular weight - with a molecular weight of up to 200 kDa and high molecular weight - with a molecular weight of more than 200 kDa [9]. These complexes are highly structurally homogeneous, so the iron they contain is released very slowly. The half-life of iron dextran is 3–4 days. The high stability of the complex and the slow release of the iron it contains make it possible to classify iron dextran complexes as clinically safe. The development of adverse events due to the use of such complexes is unlikely. However, due to their high molecular weight, these complexes may in rare cases cause allergic reactions. The reactogenicity of iron dextran complexes also depends on the route of administration. With intravenous administration, reactions are observed more often. It should be noted that with increasing molecular weight, the reactogenicity of iron dextran complexes increases when administered intravenously [9]. Iron dextrins lack this [10].
Type 2 complexes:
iron complexes of medium stability, such as iron(III) hydroxide sucrose complex with a molecular weight of 30 to 100 kDa. The half-life of these complexes is about six hours. Iron is predominantly delivered to the reticuloendothelial system (RES) and liver by transferrin and apoferritin, and is also found in the kidneys and bone marrow. Iron is quickly metabolized and becomes available for erythropoiesis. When the usual therapeutic dose of iron is administered slowly by injection or infusion, the transport system is not overloaded and free iron ions do not enter the bloodstream. The stability of the complex and the distribution of iron make this group of iron complexes relatively safe. In addition, since the complexes do not contain biological polymers, anaphylactoid reactions are quite rare [9, 11].
Type 3 complexes:
labile and weak iron complexes with a molecular weight of less than 50 kDa, for example, iron(III) gluconate, iron(III) citrate and iron(III) sorbitol. The use of ferrous gluconate in doses that include complexes of types 1 and 2 causes severe and extensive liver necrosis. Iron is found not only in the RES but also in the liver parenchyma, which leads to free radical-induced lipid peroxidation. Ferrous citrate and ferric sorbitol have a very low molecular weight (about 8.7 kDa) and are rapidly excreted by the kidneys. Therefore, very small amounts of iron can be taken up by endogenous iron-binding proteins.
Type 4 complexes
combine at least two complexes of different types (for example, iron gluconate + iron sucrose or iron citrate + iron sorbitol + iron dextrin); release iron, which can be captured by all types of proteins; communication with transferrin or apoferritin is possible only when very small doses are used. Excess iron is captured by proteins such as albumin and metabolized.
If the recommended dose is observed, drugs of type 1 and especially type 2 are optimal for intravenous administration due to their biochemical characteristics. Type 3 complexes should be administered predominantly intramuscularly, since when administered intravenously they are quickly excreted by the kidneys due to their low molecular weight. However, even with intramuscular administration, the absorption of complexes of this type can vary significantly. As a consequence, there is a risk of local and systemic adverse events, such as pain, necrosis, discoloration of the skin at the injection site. Complexes of types 3 and 4 are characterized mainly by toxic damage to cells. These complexes are more likely to cause toxic side effects than others. High renal excretion excludes the possibility of recommending them for clinical use [10, 12].
Due to the unreliability of type 3 and 4 complexes and the mechanisms of iron distribution during use, none of the complexes can be characterized as clinically safe. Even when very small doses are used, toxic reactions are possible. Intravenous use of these complexes is not recommended.
The improvement of iron preparations, including parenteral ones, has led to the fact that the acute toxicity of drugs, determined by LD50, has significantly decreased and exceeds the limits of therapeutic doses (Table 1) [13].
Anaphylactoid reactions
When using high molecular weight iron dextran, anaphylactoid reactions were observed in 0.6–2.3% of cases [14], low molecular weight iron dextran - in less than one case per 10,000 [15], sucrose iron complex - in 0.0046% of cases (17 cases per 367,000 patients, or 0.46 cases per 10,000) [7].
Types of intravenous iron supplementation
We list the types of intravenous administration of iron supplements:
- intravenous injection: 100 mg of iron, in a stream, slowly, over at least 5 minutes;
- intravenous infusion: 100–200 mg of iron, drip, in 100–200 ml of saline, for at least 15 to 60 minutes;
- intravenous single infusion of a total dose of iron with the introduction of 500–1000 mg of iron.
Indications for the use of parenteral iron supplements
Indications for the use of parenteral iron supplements, including intravenous ones, are:
- severe forms of iron deficiency anemia (IDA) (less than 3% of patients);
- ineffectiveness or intolerance of oral iron supplements;
- the presence of peptic ulcer and operations on the gastrointestinal tract, even in history;
- the need to quickly saturate the body with iron.
Treatment with parenteral iron should be safe. To do this you should:
- use parenteral drugs only when indicated;
- be sure to use a test dose if indicated in the instructions for use of the drug;
- use modern iron supplements;
- do not exceed the total iron deficiency in the body, calculated according to the formula;
- do not exceed the transferrin iron saturation coefficient (TIS).
The history of the creation of the sucrose hydroxide complex is of interest. In 1950, a sucrose hydroxide complex for intravenous administration was developed in the laboratory of Professor K. Hausmann. This drug is called Hausmann's iron (Ferrum Hausmann). Later, on the basis of the laboratory of Professor K. Hausmann, it was created (Switzerland).
Iron sucrose hydroxide complex is a high molecular weight complex (molecular weight 45–50 kDa) consisting of a centrally located core of ferric hydroxide surrounded by sucrose molecules. The structure of the complex is similar to the molecule of serum ferritin (SF), a natural protein (metalloprotein) responsible for storing iron in the body, but instead of a protein shell surrounding the core, a polysaccharide shell is used. This is believed to have reduced the immunogenicity of the molecule [7].
Modern iron preparations for intravenous administration are presented in table. 2.
New molecule – iron carboxymaltose
After the creation of the hydroxide sucrose complex, it was shown that using various polysaccharides as a shell in the molecule of an iron preparation, it is possible to create drugs with different properties. Thus, polymaltose hydroxide complex for oral administration and iron carboxymaltose for intravenous administration were obtained (see figure).
The complex consists of 1000 iron atoms with a high iron content of about 27%, the molecular weight of the complex is approximately 150 kDa. This structure of the molecule explains certain properties. At a physiological pH of about 7.0, iron hydroxide is generally insoluble. In combination with organic molecules such as carboxymaltose, it can enter the solution in the form of colloidal particles. Most iron oxides and hydroxides in the human body are located in the SF core. The matrix of the iron hydroxide preparation provides the same solubility as that of SF. After iron is absorbed by macrophages, carboxymaltose is transferred to the SF. The period of release of mononuclear iron hydroxides from carboxymaltose ranges from 7 to 21 hours [16].
Iron carboxymaltose is registered in Russia, has registration number LSR-008848/10-300810 and the trade name Ferinject®. Manufacturer of the drug – (Switzerland). The drug is available in bottles of 2 or 10 ml (100 or 500 mg of the drug, respectively). Intravenous, slow, jet or drip administration is allowed. The indication for the use of Ferinject® is iron deficiency anemia when oral iron supplements are ineffective or cannot be used [16]. Contraindications to the use of the drug Ferinject® are anemia not associated with iron deficiency, iron overload or impaired iron utilization, hypersensitivity to any of the components of the drug, pregnancy in the first trimester, age under 14 years [16].
Features of the Ferinject® drug are that there is no need to use a test dose, the possibility of administration within 15 minutes, and the use of up to 1000 mg of the drug once a week.
The drug Ferinject® is used in the treatment of IDA (any), anemia in inflammatory bowel diseases (Crohn's disease, ulcerative colitis), chronic kidney disease, obstetric and gynecological situations, heart failure, anemia caused by antitumor therapy.
The effectiveness and safety of intravenous administration of iron carboxymaltose (Ferinject®) was studied. The meta-analysis included 14 randomized controlled trials [17]. According to randomization, patients with anemia, including IDA, and various diseases (chronic kidney disease, inflammatory bowel disease, heart failure, obstetric and gynecological situations) received iron carboxymaltose (n = 2348), oral iron supplements (n = 832), intravenous iron sucrose preparation (n = 384) or placebo (n = 762). Ferinject® was administered at a dose calculated according to the formula, but not more than 1000 mg per week. At the end of the study, in the group of patients receiving Ferinject®, the increase in hemoglobin concentration, SF and NTG concentrations was greater than in the group of patients receiving oral iron supplements - by an average of 4.8 g/l (95% confidence interval (CI) 33 –63), 163 μg/L (95% CI 153–173) and 5.3% (95% CI 3.7–6.8), respectively. The incidence of constipation, diarrhea, nausea and vomiting with Ferinject® was significantly less than with oral iron supplementation.
The use of intravenous iron preparations is fully consistent with the development of modern transfusion medicine, which over its rich history has gone through three stages: stage 1 - whole blood transfusion, stage 2 - component hemotherapy using separately red blood cells, platelets, leukocytes, plasma, blood substitutes, stage 3 - drug hemotherapy with the refusal (if possible) of cellular substrates and the use of EPO, colony-stimulating factors, intravenous iron preparations, modified hemoglobin, perfluorane [2, 3].
Conclusion
The evolution of intravenous iron preparations has contributed to the emergence of highly effective and safe drugs, and developments in recent years have made it possible to simultaneously administer all the missing amounts of iron in the body. There is convincing evidence that intravenous administration of iron carboxymaltose is effective in the treatment of anemia in many diseases. The incidence of adverse events such as constipation, diarrhea, nausea, and vomiting was significantly lower with the administration of iron carboxymaltose than with oral iron supplements. The advantages of Ferinject® are the possibility of using high doses, rapid simultaneous administration, and the absence of the need to use a test dose. In modern accompanying therapy, it has become possible to correct anemia with the help of a new generation iron preparation – iron carboxymaltose.
pharmachologic effect
A remedy for the treatment of iron deficiency conditions. The polynuclear iron(III) hydroxide centers are surrounded on the outside by many non-covalently bound sucrose molecules. As a result, a complex is formed, the molecular weight of which is approximately 43 kDa, as a result of which it is impossible to excrete it unchanged by the kidneys. This complex is stable and does not release iron ions under physiological conditions. The iron in this complex is bound to structures similar to natural ferritin.
Pharmacokinetics
After a single intravenous administration of a dose containing 100 mg of iron, Cmax of iron, on average 538 µmol, is achieved 10 minutes after injection. The Vd of the central chamber almost completely corresponds to the volume of the serum - about 3 liters. Vd at steady state is approximately 8 L (which indicates a low distribution of iron in body fluids). Due to the low stability of iron saccharate compared to transferrin, competitive iron metabolism is observed in favor of transferrin. As a result, about 31 mg of iron (III) is transferred in 24 hours. T1/2 - about 6 hours. In the first 4 hours, less than 5% of iron from the total clearance is excreted by the kidneys. After 24 hours, the serum iron level returns to its original (pre-administration) value, and approximately 75% of sucrose leaves the vascular bed.
Side effects
When used correctly, Venofer is well tolerated by patients. During therapy with Venofer, patients may develop the following undesirable effects:
From the central nervous system: loss of consciousness, dizziness, paresthesia, headache, asthenia.
From the heart and blood vessels: palpitations, tachycardia, arterial hypotension, feeling of heat, collapse, facial hyperemia, edema.
From the gastrointestinal tract: discomfort and pain in the epigastric region, nausea, vomiting, changes in taste, diarrhea.
Allergic reactions: erythema, itching, urticaria, hyperpigmentation, increased sweating, bronchospasm. In addition, the development of angioedema and anaphylactic shock is possible (the first administration of the drug should be carried out under the supervision of a physician if means for resuscitation are available).
Others: shortness of breath, pain in the joints and muscles, swelling of the joints, pain in the limbs and chest, local reactions upon administration (hyperemia, itching and swelling of the tissues), pallor of the skin, hyperthermia, chills.
Interaction with other drugs
The combined use of Venofer with oral iron preparations is prohibited. Taking an oral form of iron is recommended no earlier than 5 days after the end of therapy with Venofer. Solution for parenteral use Venofer can be mixed only with 0.9% sodium chloride solution. It is prohibited to mix the drug in the same syringe with other drugs for parenteral use. Venofer is compatible with containers made of glass, polyvinyl chloride and polyethylene. Compatibility with other substances has not been studied.
Overdose
It is practically unlikely, since the absorption of iron from the drug occurs through active transport, and the level of absorption depends on the level of iron deficiency in the body and is impossible above the physiological norm. Excess of the drug that is not absorbed is excreted from the body with feces. The possibility of irritation of the mucous membranes of the gastrointestinal tract due to an excess of unabsorbed drug cannot be ruled out. To eliminate this phenomenon, it is possible to prescribe mucous decoctions or cytoprotective gels.
Contraindications
Venofer is not prescribed to patients with individual hypersensitivity to the ingredients included in the solution, as well as to other parenteral iron preparations. Venofer is not used to treat patients with hemochromatosis, hemosiderosis, impaired iron excretion, as well as anemia that is not associated with iron deficiency. It is prohibited to prescribe parenteral iron (III) solution in the first trimester of pregnancy. It is recommended to exercise caution when prescribing Venofer to patients suffering from bronchial asthma, polyvalent allergies, eczema, folic acid deficiency, and liver dysfunction. Venofer is prescribed with caution to patients suffering from acute and chronic infectious diseases in which plasma ferritin levels are elevated. Administration of the drug Venofer to children is allowed only under the close supervision of a physician and in a dose of no more than 0.15 ml of parenteral solution per 1 kg of body weight once every 72 hours.
general characteristics
Chemical name: iron oxide saccharate;
Basic physical and chemical properties: transparent liquid of red-brown color, with a vanilla odor
Composition: active ingredient: 100 g of solution contains iron oxide saccharate - 7.39 g;
Excipients: refined sugar, vanillin, ethyl alcohol, purified water.
Release form. Solution for oral use.
Pharmacological group. Ferric iron preparations. ATS CODE: В03А В02.