Diclofenac gel
Dosage form
Gel for external use
Composition per 100 g:
Active substance
: diclofenac sodium – 1.00 g.
Excipients
: 2-propanol – 45.00 g, macrogol 7 glyceryl cocoate – 6.00 g, hypromellose 4000 – 3.00 g, Scots pine oil – 0.15 g, lavender oil – 0.05 g, purified water – up to 100 .00 g.
Description
Colorless or with a slight yellowish tint, transparent gel with a characteristic odor.
Pharmacotherapeutic group
Non-steroidal anti-inflammatory drug (NSAID).
ATX code: M02AA15.
Pharmacological properties
Pharmacodynamics
The active component diclofenac is a non-steroidal anti-inflammatory drug with pronounced analgesic, anti-inflammatory and antipyretic properties. Indiscriminately inhibiting cyclooxygenase types 1 and 2, it disrupts the metabolism of arachidonic acid.
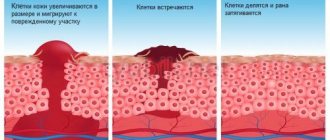
Diclofenac is used to eliminate pain and inflammation in joints, muscles and ligaments of traumatic or rheumatic origin, helping to reduce pain and swelling associated with the inflammatory process, increasing joint mobility.
Thanks to its hydroalcoholic base, Diclofenac has a calming and cooling effect.
Pharmacokinetics
The amount of diclofenac absorbed through the skin is proportional to the area of the treated surface and depends on both the total dose of the drug applied and the degree of skin hydration. After applying Diclofenac, gel for external use 1% (4 applications per day) to a skin surface area of 400 cm2, the concentration of the active substance in the plasma corresponds to its concentration when using 2% diclofenac gel (2 applications per day). On day 7, the relative bioavailability of the drug (AUC ratio) is 4.5% (for an equivalent dose of diclofenac sodium salt). When wearing a moisture-permeable dressing, suction did not change. The concentration of diclofenac in plasma, synovial membrane and synovial fluid was measured when the drug was applied to the area of the affected joint. Maximum plasma concentrations were approximately 100 times lower than after oral administration of the same amount of diclofenac. 99.7% of diclofenac is bound to plasma proteins, mainly to albumin (99.4%).
Diclofenac is preferentially distributed and retained deep in tissues prone to inflammation, such as joints, where its concentration is 20 times higher than in plasma.
The metabolism of diclofenac is carried out partly by glucuronidation of the unchanged molecule, but mainly through single and multiple hydroxylation, which leads to the formation of several phenolic metabolites, most of which are converted to glucuronide conjugates. Two phenolic metabolites are biologically active, but to a much lesser extent than diclofenac.
The total systemic plasma clearance of diclofenac is 263±56 ml/min. The terminal half-life is 1-2 hours. The half-life of metabolites, including two pharmacologically active ones, is also short and amounts to 1-3 hours. One of the metabolites (3′-hydroxy-4′-methoxydiclofenac) has a longer half-life, however, this the metabolite is completely inactive. Most of diclofenac and its metabolites are excreted in the urine.
Indications for use
• Back pain due to inflammatory and degenerative diseases of the spine (sciatica, osteoarthritis, lumbago, sciatica),
• Pain in the joints (joints of the fingers, knees, etc.) with osteoarthritis,
• Muscle pain (due to sprains, strains, bruises, injuries),
• Inflammation and swelling of soft tissues and joints due to injuries and rheumatic diseases (tenosynovitis, bursitis, lesions of periarticular tissues, wrist syndrome).
Contraindications
Hypersensitivity to diclofenac or other components of the drug; tendency to develop attacks of bronchial asthma, skin rashes or acute rhinitis when using acetylsalicylic acid or other NSAIDs; pregnancy (III trimester), breastfeeding; children's age (up to 12 years); violation of the integrity of the skin at the intended site of application.
Carefully
Hepatic porphyria (exacerbation), erosive and ulcerative lesions of the gastrointestinal tract, severe dysfunction of the liver and kidneys, blood clotting disorders (including hemophilia, prolonged bleeding time, bleeding tendency), chronic heart failure, bronchial asthma, old age, pregnancy (I and II trimester).
Use during pregnancy and breastfeeding
Due to the lack of data on the use of Diclofenac in pregnant women, the use of the drug during the first and second trimesters of pregnancy is recommended only as prescribed by a doctor, weighing the benefits for the mother and the risk for the fetus.
The drug is contraindicated in the third trimester of pregnancy due to the possibility of decreased uterine tone, impaired fetal renal function with subsequent development of oligohydramnios and/or premature closure of the fetal ductus arteriosus.
Due to the lack of data on the penetration of Diclofenac into breast milk, the drug is not recommended for use during breastfeeding. If it is still necessary to use the drug, it should not be applied to the mammary glands or large surface areas of the skin and should not be used for a long time.
There are no data on the use of Diclofenac and its effect on fertility in humans.
Directions for use and doses
Externally.
For adults and children over 12 years of age, the drug is applied to the skin 2 times a day (every 12 hours: preferably morning and evening), lightly rubbing into the skin.
The required amount of the drug depends on the size of the painful area. A single dose of the drug - 4-8 g (which is comparable in volume to twice the size of a cherry or walnut) is enough to treat an area of 400-800 cm2. If your hands are not the area where pain is localized, then after applying the drug they should be washed. The duration of treatment depends on the indications and the observed effect. The gel should not be used for more than 14 days for post-traumatic inflammation and rheumatic diseases of soft tissues without a doctor’s recommendation. If after 7 days of use the therapeutic effect is not observed or the condition worsens, you should consult a doctor.
To remove the protective membrane, use the screw cap as a key (the recess with protrusions on the outside of the cap). Align the indentation on the outside of the cap with the shaped protective membrane of the tube and turn. The membrane should separate from the tube.
The tubes can have either a regular cap (round shape) or an innovative cap (triangular shape), which is especially convenient for use when the mobility of the hand joints is limited due to osteoarthritis or other joint diseases or injuries.
Side effect
Classification of the frequency of occurrence of adverse reactions:
very often (> 1/10); often (> 1/100, < 1/10); uncommon (> 1/1000, < 1/100); rare (> 1/10000, < 1/1000); very rare (< 1/10000), including isolated reports.
Infectious and parasitic diseases:
Very rare: pustular rash.
Immune system disorders:
Very rare: hypersensitivity reactions (including urticaria), angioedema. Respiratory, thoracic and mediastinal disorders: Very rare: asthma.
Disorders of the skin and subcutaneous tissues:
Common: dermatitis (including contact dermatitis), rash, erythema, eczema, itching.
Rarely: bullous dermatitis.
Very rare: photosensitivity reactions.
If any side effects occur, including those not indicated in the instructions, you must stop using the drug and consult a doctor.
Overdose
Due to the low systemic absorption when applying the gel, overdose is unlikely.
In case of accidental ingestion, systemic adverse reactions may develop. Treatment of overdose due to accidental ingestion: gastric lavage, induction of vomiting, activated charcoal, symptomatic therapy. Dialysis and forced diuresis are not effective due to the high degree of binding of diclofenac to plasma proteins (about 99%).
Interaction with other drugs
The drug may enhance the effect of drugs that cause photosensitivity. Clinically significant interactions with other drugs have not been described.
special instructions
Diclofenac should be applied only to intact skin, avoiding contact with open wounds. The drug should not come into contact with the mouth, eyes or mucous membranes. After applying the drug, a bandage may be applied, but airtight occlusive dressings should not be applied. If a skin rash develops after application of the drug, its use should be discontinued.
The effect of the drug on the ability to drive vehicles and machinery
Does not affect.
Release form
Gel for external use 1%.
5, 10, 15, 20, 25, 30, 35, 40, 50, 60, 70, 80, 90, 100 g in orange glass jars with a triangular rim and a lid that is tensioned with a sealing element.
5, 10, 15, 20, 25, 30, 35, 40, 50, 60, 70, 80, 90, 100 g in polymer jars complete with lids or in polyethylene terephthalate jars with closures.
20, 25, 30, 35, 40, 50, 60, 70, 80, 100 g in aluminum tubes, coated with BF-2 varnish, with caps made of high-density polyethylene or in polymer tubes with polyethylene screw caps.
Each jar and tube, along with instructions for use, is placed in a cardboard pack.
Storage conditions
At a temperature not higher than 25 °C.
Keep out of the reach of children.
Best before date
3 years.
Do not use after the expiration date stated on the packaging.
Vacation conditions
Available without a prescription.
Diclofenac ointment for external use 1% tube 30g
Compound
Active substance: diclofenac sodium - 1 g.
Excipients: isopropanol - 45 g, macrogol 7 glyceryl cocoate - 6 g, hypromellose 4000 (in terms of dry matter) - 3 g, Scots pine oil - 0.15 g, lavender oil - 0.05 g , purified water - up to 100 g.
Pharmacokinetics
The amount of diclofenac absorbed through the skin is proportional to the area of the treated surface and depends on both the total dose of the drug applied and the degree of skin hydration. The binding of diclofenac to plasma proteins is 99.7%, mainly with albumin (99.4%). Diclofenac is preferentially distributed and retained deep in tissues prone to inflammation, such as joints, where its concentration is 20 times higher than in plasma.
The metabolism of diclofenac is carried out partly by glucuronidation of the unchanged molecule, but mainly through single and multiple hydroxylation, which leads to the formation of several phenolic metabolites, most of which are converted to glucuronide conjugates. Two phenolic metabolites are biologically active, but to a much lesser extent than diclofenac.
The total systemic plasma clearance of diclofenac is 263±56 ml/min.
The final T1/2 is 1-2 hours. The T1/2 of metabolites, including two pharmacologically active ones, is also short-lived and is 1-3 hours. One of the metabolites (3′-hydroxy-4′-methoxydiclofenac) has a longer T1/2, however, this metabolite is completely inactive. Most of diclofenac and its metabolites are excreted in the urine.
Indications for use
- inflammatory diseases of the musculoskeletal system (rheumatoid arthritis, psoriatic arthritis, juvenile chronic arthritis, ankylosing spondylitis, gouty arthritis);
- rheumatic lesions of soft tissues (tenosynovitis, bursitis, damage to periarticular tissues);
- degenerative diseases of the musculoskeletal system (deforming osteoarthritis, osteochondrosis);
- post-traumatic inflammation of tendons, ligaments, muscles and joints (as a result of injuries, stress and bruises);
- pain and swelling associated with diseases of muscles and joints (lumbago, sciatica, neuralgia, myalgia, tendinitis).
Intended for symptomatic therapy, reducing pain and inflammation at the time of use, does not affect the progression of the disease.
Contraindications
- “Aspirin triad” (attacks of bronchial asthma, urticaria and acute rhinitis when taking acetylsalicylic acid or other NSAIDs);
- violation of the integrity of the skin at the site of application of the drug;
- III trimester of pregnancy;
- lactation period (breastfeeding);
- children up to 6 years of age and older, depending on the drug used;
- hypersensitivity to diclofenac, other NSAIDs or to any of the excipients of the drug used.
With caution: hepatic porphyria (in the acute phase); erosive and ulcerative lesions of the gastrointestinal tract; severe dysfunction of the liver and kidneys; chronic heart failure; bronchial asthma; bleeding disorders (including hemophilia, prolongation of bleeding time, bleeding tendency); I and II trimesters of pregnancy; elderly patients.
Directions for use and doses
Externally.
For adults and children over 12 years of age, the drug is applied to the skin 2-3 times a day, at a dose of 2-4 g (about 4 cm with the neck of the tube completely open). Apply a thin layer to the skin above the inflammation and rub in lightly.
For children from 6 to 12 years old, use no more than 2 times a day, a single dose of the drug is up to 1 g (about 2 cm with the neck of the tube completely open). The maximum daily dose of the gel should not exceed 8 g.
The course of treatment is no more than 14 days. The need for longer-term use of the drug is determined by the doctor. After applying the gel, you must wash your hands.
Use in minimally effective doses for a minimally short course.
Storage conditions
In a place protected from light at a temperature not exceeding 25° C.
Keep out of the reach of children.
Best before date
3 years. Do not use after the expiration date.
special instructions
Apply externally only. The gel can only be applied to intact areas of the skin. After applying the gel, you should not apply an occlusive dressing. Before use, patients with gastric and duodenal ulcers, impaired liver, kidney or hematopoietic function, as well as concomitant use of other non-steroidal anti-inflammatory drugs, should consult a doctor. When using the drug together with other dosage forms of diclofenac, the maximum daily dose should be taken into account.
With long-term use and/or application to large surfaces, systemic side effects may develop due to the resorptive effect. Avoid contact of the drug with the eyes, mucous membranes or open wounds.
Description
Non-steroidal anti-inflammatory drug (NSAID).
Dosage form
Gel for external use 1% colorless or with a slight yellowish tint, transparent, odorless.
Use in children
Not recommended for use in children under 6 years of age.
Pharmacodynamics
NSAID for external use, a derivative of phenylacetic acid. Has anti-inflammatory and analgesic effects. The mechanism of action is due to inhibition of the activity of COX-1 and COX-2, which leads to disruption of the metabolism of arachidonic acid and the synthesis of prostaglandins, which are the main link in the development of inflammation.
When used externally, it leads to the disappearance or weakening of pain at the site of application, reduces pain in the joints at rest and during movement, as well as morning stiffness and swelling of the joints. Helps increase range of motion in affected joints.
Side effects
The incidence of side effects is classified according to the recommendations of the World Health Organization: characterized as very common - at least 10%; often - at least 1%, but less than 10%; infrequently - not less than 0.1%, but less than 1%; rarely - not less than 0.01%, but less than <0.1%; very rare, including individual messages - less than 0.01%.
Local reactions: infrequently - exudative erythema multiforme (including Stevens-Johnson syndrome), eczema, very rarely - contact dermatitis (itching, hyperemia, swelling of the treated skin area, papular-vesicular rashes, peeling).
When applying the gel to large surfaces of the skin over a long period of time, systemic side effects of diclofenac may develop.
Systemic reactions: rarely - generalized skin rash, allergic reactions (urticaria, angioedema, bronchospastic reactions), rarely - photosensitivity, very rarely - anaphylactic reactions (including shock).
Use during pregnancy and breastfeeding
Use is contraindicated in the third trimester of pregnancy due to the possibility of decreased uterine tone and/or premature closure of the fetal ductus arteriosus.
Use in the first and second trimesters of pregnancy is possible in cases where the potential benefit to the mother outweighs the potential risk to the fetus or infant.
Use during lactation (breastfeeding) is contraindicated.
Interaction
The use of the drug with other non-steroidal anti-inflammatory drugs is not recommended.
Diclofenac may enhance the effect of drugs that cause photosensitivity. Clinically significant interactions with other drugs have not been described.
Overdose
Due to the low systemic absorption upon application of the gel, overdose is unlikely.
In case of accidental ingestion, systemic adverse reactions may develop. Symptoms: nausea and vomiting.
Treatment: gastric lavage, induction of vomiting, activated charcoal, forced diuresis, symptomatic therapy.
Hemodialysis is not effective due to the high degree of protein binding of diclofenac (about 99%).
Impact on the ability to drive vehicles and operate machinery
The drug does not affect the ability to drive a car or use other mechanical means.
Diclofenac and pregnancy: consequences and complications
Diclofenac may have adverse effects on the health of a pregnant woman and her unborn child.
Research by specialists has shown that the use of diclofenac in the first and second trimesters of pregnancy is permissible only in cases where the therapeutic benefit to the mother outweighs the potential danger to the child, which allows us to draw a negative conclusion whether diclofenac can be taken by pregnant women.
Taking diclofenac during the third trimester of pregnancy (especially after 30 weeks) is unacceptable, as it may be accompanied by the following consequences:
- premature closure of the fetal ductus arteriosus. This process occurs as a result of an increase in oxygen content in the blood of newborns. Intrauterine closure of the ductus arteriosus can lead to right ventricular failure;
- fetal renal failure;
- suppression of platelet aggregation, which can lead to bleeding;
- suppression of uterine contractility, which can cause a delay in labor.
Due to the inhibition of enzymes and mediators (cyclooxygenase and prostaglandins), diclofenac can negatively affect female fertility (the ability to bear children). As a result, diclofenac should be discontinued when planning pregnancy, or in patients undergoing examination for infertility.
Thus, clinical pharmacist and research initiator at Oslo University Hospital Rikshospitalet, Katerina Nezvalova-Henriksen, conducted an experiment in which she found out the effect of diclofenac on the course and outcome of pregnancy. This study, which included 6,511 pregnant women, found no effect on infant survival, birth defects, or structural heart defects. However, diclofenac use in the second trimester was significantly associated with low birth weight. Also, the use of diclofenac in the third trimester could lead to vaginal bleeding in women.
The study results suggested that the increased risk of low birth weight may have been due in part to underlying inflammatory conditions and was reassuringly similar to the expected baseline risk of low birth weight.