What properties does Oseltamivir have?
The drug was recommended by the FDA in 1999 for the treatment of acute respiratory diseases, and in 2009 it was approved by WHO. Oseltamivir is more often prescribed to people at risk of severe complications. The results of several studies have shown that in flu patients without chronic diseases, symptoms decrease faster in the first days than in other categories of patients. However, evidence-based medicine has not confirmed that taking the medicine prevents the transmission of infection between people. Relief comes due to the substance’s ability to effectively reduce acute symptoms, but not kill bacteria. Indications for use: influenza type A and B.
Oseltamivir - instructions for use and dosage
During an epidemic, it is recommended to take:
- for prevention - 75 mg 1-2 times a day for 1.5 months;
- when the first symptoms of flu appear - 2 rubles. 75 mg for 5 days;
- children over 8 years old - 1 time/day.
Only adolescents over 12 years of age, as well as adults, are prescribed, if necessary, to take capsules twice a day - morning and evening. Until the age of eight, doctors recommend a suspension.
When the dosage is increased to 150 mg, no improved effect is observed. Take regardless of food intake.
Oseltamivir capsules 75 mg No. 10 Akrikhin
Action
Oseltamivir is a prodrug that, when taken orally, undergoes hydrolysis and is converted into the active form, oseltamivir carboxylate.
The mechanism of action of oseltamivir carboxylate is associated with the inhibition of neuraminidase of influenza viruses type A and B. Neuraminidase, a surface glycoprotein of the influenza virus, is one of the key enzymes involved in the replication of influenza viruses A and B. There are 9 known antigenic subtypes of neuraminidase of influenza virus type A - N1, N2 etc., which, along with 16 antigenic subtypes of hemagglutinin - H1, H2, etc., determine different strains of the same type of virus. Several strains of influenza A virus with hemagglutinin 1–5 and neuraminidase 1 and 2 are simultaneously circulating in the human population, the main of which are H3N2 and H1N1. When neuraminidase is inhibited, the ability of viral particles to penetrate into the cell, as well as the release of virions from the infected cell, is impaired, which leads to limiting the spread of infection in the body.
The in vitro antiviral activity of oseltamivir carboxylate was assessed in cell cultures using laboratory strains and clinical isolates of influenza virus. The concentrations of oseltamivir carboxylate required to inhibit influenza virus have been shown to be highly variable and dependent on the testing method used and the virus tested. IC50 and IC90 values (concentrations required to inhibit enzyme activity by 50 and 90%) range from 0.0008 to >35 μM and 0.004 to >100 μM, respectively (1 μM = 0.284 μg/ml). The relationship between in vitro antiviral activity in cell culture and inhibition of viral replication in humans has not been established.
Resistance. Influenza A virus isolates with reduced sensitivity to oseltamivir carboxylate were passaged in vitro in the presence of increasing concentrations of oseltamivir carboxylate. Genetic analysis of these isolates showed that reduced sensitivity to oseltamivir carboxylate is associated with mutations leading to changes in the amino acids of both viral neuraminidase and hemagglutinin. The mutations that conferred resistance in vitro were I222T and H274Y of influenza A virus N1 neuraminidase and I222T and R292K of influenza A virus N2 neuraminidase. For influenza A virus N9 neuraminidase, the typical mutations in birds were E119V, R292K and R305Q; for hemagglutinin of the influenza A virus H3N2 - mutations A28T and R124M, for hemagglutinin of the reassortant human/avian H1N9 virus - mutation H154Q (reassortment is the construction of the genome of a daughter virus from the genomes of different parents, in this case the avian influenza virus and the human influenza virus).
A clinical study of resistance (naturally acquired) in influenza virus-infected patients showed that 1.3% (4/301) of post-treatment clinical isolates from adults and adolescents and 8.6% (9/105) - from children 1–12 years old, varieties with reduced sensitivity of the neuraminidase virus to oseltamivir carboxylate in vitro were identified. The influenza A virus mutations that resulted in decreased sensitivity were H274Y in neuraminidase N1 and E119V and R292K in neuraminidase N2. There is insufficient information to fully characterize the risk of resistance to oseltamivir phosphate during clinical use.
With post-exposure and seasonal prophylactic use of oseltamivir phosphate, resistance determination was limited due to the low overall incidence of viral infection.
Cross resistance. Cross-resistance has been observed between zanamivir-resistant mutant strains and oseltamivir-resistant mutant influenza strains in vitro, the frequency of which could not be determined.
Immune response. No interaction studies have been conducted with influenza vaccine. In studies with natural and experimental influenza infection, treatment with oseltamivir phosphate did not affect normal antibody production in response to infection.
Carcinogenicity, mutagenicity, effect on fertility
Long-term studies assessing the carcinogenic effect of oseltamivir have not yet been completed. However, a 26-week skin carcinogenicity study of oseltamivir carboxylate in FVB/Tg.AC transgenic mice showed negative results. Animals received 40, 140, 400, or 780 mg/kg/day in two divided doses. The highest dose reflected the maximum possible dose based on the solubility of the substance in the corresponding solvent. The control (tetradecanoylphorbol-13 acetate, 2.5 mg per dose 3 times a week) gave a positive result (inducing carcinogenesis).
No mutagenic properties of oseltamivir were detected in the Ames test, a test for chromosomal aberrations on human lymphocytes with or without metabolic activation, or in a micronucleus test in mice. A positive result was obtained in the cell transformation test on SHE (Syrian Hamster Embryo) cells. Oseltamivir carboxylate was not mutagenic in the Ames test, a test on L5178Y mouse lymphoma cells with or without metabolic activation; the test on SHE cells was negative.
In a reproductive study in rats, female rats were administered oseltamivir phosphate at doses of 50, 250, and 1500 mg/kg/day for 2 weeks before mating, during mating, and until day 6 of pregnancy; male rats received oseltamivir for 4 weeks before mating, during mating, and for 2 weeks after mating. There were no indications of the effect of any of the doses studied on fertility, mating, or early embryonic development. The highest dose was approximately 100 times the human systemic exposure (AUC0–24 h) of oseltamivir carboxylate.
Toxicology in animals
In a two-week study, administration of oseltamivir phosphate at a single dose of 1000 mg/kg to 7-day-old rat pups resulted in death due to unusually high exposure to the prodrug. However, in 14-day-old rat pups, no deaths or other significant adverse effects were observed at doses of 2000 mg/kg. Subsequent studies showed that in 7-day-old deceased rat pups, prodrug concentrations in the brain were approximately 1500 times higher than those in the brains of adult rats that received the same dose of 1000 mg/kg orally and in which the level of the active metabolite was approximately 3 times higher. higher. Plasma levels of the prodrug were 10 times higher in 7-day-old rat pups compared to adult animals. These observations suggest that oseltamivir concentrations in rat brain decrease with increasing age and most likely reflect the stage of BBB formation. At a dose of 500 mg/kg/day, no adverse effects were observed in 7-day and 21-day rat pups; at this dose, prodrug exposure was approximately 800 times higher than that calculated for a one-year-old child.
Pharmacokinetics
Oseltamivir is rapidly absorbed from the gastrointestinal tract after oral administration of oseltamivir phosphate and is extensively converted to oseltamivir carboxylate, primarily by hepatic esterases. At least 75% of the dose taken enters the systemic circulation in the form of oseltamivir carboxylate, less than 5% - unchanged. After repeated oral administration of 75 mg oseltamivir phosphate capsules 2 times a day (n=20), the mean Cmax values of oseltamivir and oseltamivir carboxylate were 65.2 and 348 ng/ml, AUC0–12 h - 112 and 2719 ng h/ml respectively. Plasma concentrations of oseltamivir carboxylate are dose proportional when administered up to 500 mg twice daily. Concomitant food intake does not have a significant effect on the Cmax of oseltamivir carboxylate (551 ng/ml when taken on an empty stomach, 441 ng/ml when taken after a meal) and AUC (6218 and 6069 ng h/ml, respectively).
The volume of distribution of oseltamivir carboxylate after intravenous administration to 24 volunteers ranged from 23 to 26 L. The binding of oseltamivir to plasma proteins is average (42%), oseltamivir carboxylate is very low (<3%).
In vitro studies have demonstrated that neither oseltamivir nor oseltamivir carboxylate are substrates or inhibitors of multifunctional cytochrome P450 oxidases.
More than 90% of absorbed oseltamivir is converted to oseltamivir carboxylate; when taken orally, T1/2 from plasma for oseltamivir is 1–3 hours. Oseltamivir carboxylate is not further metabolized and is excreted by the kidneys (more than 99%); T1/2 from plasma for oseltamivir carboxylate is 6–10 hours. Renal clearance (18.8 L/h) exceeds the glomerular filtration rate (7.5 L/h), indicating elimination by tubular secretion, in addition to glomerular filtration . Less than 20% of the ingested radioactive dose is eliminated in feces.
Dependence of pharmacokinetic parameters on certain factors
Renal dysfunction. When prescribing oseltamivir phosphate 100 mg 2 times a day for 5 days to patients with varying degrees of renal impairment, exposure (AUC) of the active metabolite was shown to be inversely proportional to the decrease in renal function.
Childhood. The pharmacokinetics of oseltamivir and oseltamivir carboxylate were studied after a single dose in children aged 5 to 16 years (n=18) and in a small number of patients aged 3 to 12 years (n=5) included in clinical trials. In young children, elimination of both the prodrug and its active metabolite was more rapid than in adult patients, resulting in lower AUC values at the same dose (in mg/kg). The apparent total clearance of oseltamivir carboxylate decreased linearly with increasing age (up to 12 years). The pharmacokinetics of oseltamivir in children over 12 years of age is similar to that of adult patients.
Elderly age. In patients 65–78 years of age, the AUC of oseltamivir carboxylate at steady state was 25–35% greater than in younger adult patients when given similar doses of oseltamivir. T1/2 values in elderly patients were comparable to those observed in young patients. Taking into account the exposure of the substance (AUC) and tolerability in elderly patients, no dose adjustment is required during treatment and prophylaxis.
Side effects
Taking Oseltamivir is sometimes accompanied by negative symptoms:
- nausea;
- vomiting;
- abdominal pain;
- dizziness;
- sore throat;
- nasal congestion;
- increased anxiety;
- general malaise;
- insomnia;
- convulsions.
The drug is prescribed with extreme caution to children. At the first side effects, it is not recommended to take it. Marketing data from 2006 in Japan indicated that psychiatric abnormalities, thought disorders and other neurocognitive changes may occur.
Brand and are there any analogues?
Oseltamivir or oseltamivir is a drug known in Russia under the trade name Tamiflu. The Latin name of the active ingredient is oseltamivir. The crystalline substance is produced in the form of a white powder and capsules for suspension. During periods of widespread illness, children are recommended to make a solution, and adults to take capsules.
Oseltamivir - instructions on how to prepare a solution
It can be drunk at any time, regardless of meals.
1 Lightly tap the closed bottle to distribute the powder evenly.
2 Pour 52 ml of boiled and cooled water into a measuring cup.
3 Add the contents to the bottle, close and shake.
4 After removing the cap, insert the adapter into the neck of the bottle.
5 Tighten as tightly as possible.
It is recommended to mark the expiration date on the bottle. It is stored at room temperature for 10 days, and in the refrigerator for up to 17. Be sure to shake the bottle before taking it. The kit contains instructions for using Oseltamivir. Using a dosing syringe, draw the required amount of liquid: 30.45 or 60 mg.
Experience with the use of oseltamivir (Tamiflu) for influenza and ARVI in children
More than half a million people die each year from influenza and its complications [1]. The most effective way to reduce morbidity is specific vaccine prevention. But since influenza viruses are capable of antigenic variability in the surface proteins hemagglutinin and neuraminidase, vaccine developers are forced to annually create new antigenic drugs, the release of which takes at least 9 months, and therefore it is impossible to guarantee a complete match between the vaccine and the circulating strain [2]. (In addition, given the emerging threat of a new pandemic strain of avian influenza (H5N1), which may appear quite suddenly, the question arises of the need to use effective etiotropic chemotherapy drugs.)
Currently, the class of anti-influenza drugs includes the adamantadines amantadine and rimantadine, whose action is aimed at M2 ion channels and the neuraminidase inhibitors oseltamivir and zanamivir [3]. However, adamantadines are currently 30–60% ineffective. Thus, over 10 years of observation, the frequency of cases of drug resistance increased from 0.4% in 1995 to 12.3% in 2004. The development of resistance to both drugs is associated with a mutation of the influenza A virus, as a result of which the function of the M2 protein ion channel was changed protected from the action of adamantadines [4, 5, 6, 7, 8].
On the contrary, the mutations necessary for the development of resistance to neuraminidase inhibitors occur in a hard-to-reach region of the neuraminidase enzyme molecule, which reduces its activity and the possibility of developing resistance, thereby determining the activity of inhibitors of this enzyme against all subtypes of viruses A and B.
The first drug in this group was zanamivir, intended for local use in the form of inhalation, but does not have a systemic effect against influenza, which led to the development of a new drug - oseltamivir (Tamiflu). According to the literature, the use of Tamiflu for influenza in adult patients reduces the severity of the disease, shortens the period of clinical manifestations, and reduces the frequency of complications requiring the use of antibacterial agents [9, 10, 11, 12, 13]. However, little information has been collected on the use of Tamiflu in children, which served as the purpose of this study to study the clinical effectiveness and safety of this drug for influenza and other respiratory infections in children.
50 children aged 12 to 15 years were observed, 25 of whom received Tamiflu (main group) and 25 received conventional treatment (control group). 34% of children were classified as frequently ill. 9 children had chronic pathology: 1 had recurrent obstructive bronchitis, 1 had bronchial asthma, 7 had chronic tonsillitis, 2 had hay fever.
Based on the totality of clinical data, influenza could be diagnosed in 16 patients of the main and 14 control groups, parainfluenza - in 9 and 11 patients, adenoviral infection - in 8 and 6 patients, respectively.
The drug Tamiflu at a dose of 75 mg was prescribed in the acute period of the disease 2 times a day no later than the first 30 hours of the disease for 5 days against the background of concomitant symptomatic therapy. From the first day of seeing a doctor, children from the control group received antihistamines, antipyretics, and mucolytic drugs, and 13 of them, in addition to symptomatic treatment, received antibiotics due to the occurrence of bacterial complications.
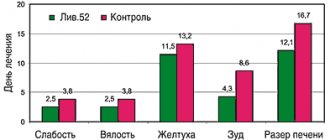
Patients treated with Tamiflu had a significantly shorter duration of clinical symptoms, regardless of the severity of ARVI (Figure a).
As can be seen from the data presented, the duration of fever and other manifestations of intoxication syndrome (malaise, loss of appetite, weakness, drowsiness, decreased physical activity) was reduced by 3 or more times; rhinitis - 2.6 times; During Tamiflu therapy, a productive cough with a sputum thinning effect appeared from the 2nd–3rd day, and in the control group - after the 4th day. The maximum clinical effect among patients with ARVI was observed in 72% of children already on the second day of taking the drug. At the same time, 67% of children showed a change in sputum density towards its dilution, while in the control group these changes were recorded only in 43% of children.
In ARVI patients from the control group with bacterial complications, the duration of fever and other manifestations of intoxication syndrome decreased by 4.4 times; duration of rhinitis - 3.2 times; A productive cough with a sputum thinning effect appeared during Tamiflu treatment on average from the 2nd day, and in the control group - from the 5th day (Figure b).
The drug was well tolerated in all children. There were no adverse reactions in the form of abdominal pain, nausea, vomiting, dyspepsia, sensation of a lump or foreign body, dryness/soreness in the throat associated with receiving Tamiflu. No allergic reactions were detected during Tamiflu therapy. In children suffering from atopic dermatitis, hay fever, and bronchial asthma, there was also no aggravation of the manifestations of the disease.
The study showed that Tamiflu is harmless, well tolerated, and effective in treating not only influenza, but also ARVI. The mechanism of clinical improvement in ARVI during treatment with Tamiflu is not entirely clear. Since during treatment with the drug (75 mg 2 times a day for 5 days), all children, regardless of the etiology of the disease, showed a significant improvement in well-being and a rapid reversal of clinical manifestations, it can be assumed that Tamiflu inhibits not only neuraminidase, but also other factors of viral pathogenicity. Undoubtedly, the clinical effect of the drug is directly related to the suppression of viral aggression, and this, as is known, leads to a decrease in the level of pro-inflammatory cytokines, the number of cells expressing CD95+, a decrease in the adhesive ability of mononuclear blood cells, activation of lymphocytes, targeted polarization of Th-0 lymphocytes in Th-1, expression on the cell membrane of HLA-DR class 11, stimulation of the phagocytic system of macrophages and neutrophils, growth and activation of cytotoxic and NK cells [14, 15, 16].
Such changes in the immune system contribute to the suppression of bacterial and fungal flora, determining the uncomplicated course of the disease.
Our data are consistent with information from other authors. Thus, according to L. Kaiser (2003), when using Tamiflu, the symptoms of the underlying disease not only resolve faster, but also reduce by 55%, compared with placebo, the frequency of secondary complications from the lower respiratory tract, as well as the number of hospitalizations. In high-risk patients, the incidence of bacterial complications requiring antibiotic therapy decreased by 34%, and the number of hospitalizations decreased by 59% [17].
According to CM Machado (2004), in patients with weakened immunity as a result of bone marrow transplantation, Tamiflu was not only highly effective, but also well tolerated [18].
The work of RJ Whitley (2001) showed the effectiveness of Tamiflu in children aged 1 year and older. In children receiving the drug, recovery occurred 36 hours earlier than in the control group. At the same time, the frequency of bacterial complications decreased by 44%, and a significant increase in forced expiratory volume in 1 s was observed in children with bronchial asthma [19].
Based on the results of our study, the following conclusions can be drawn.
- Oseltamivir (Tamiflu) can be considered the drug of choice for the treatment of influenza and other respiratory infections.
- Modern etiotropic therapy of influenza and acute respiratory viral infections with the drug Tamiflu helps reduce the duration of fever, intoxication phenomena, as well as the timing of relief of catarrhal symptoms.
- With systemic therapy with oseltamivir (Tamiflu), the development of airway obstruction and bacterial complications is not observed, which allows one to avoid hospitalization and refuse to prescribe antibacterial therapy.
- No cases of hypersensitivity to Tamiflu have been identified; the drug is well tolerated and has no contraindications.
- Oseltamivir (Tamiflu) for influenza and other respiratory infections should be prescribed 75 mg 2 times a day for 5 days in children over 12 years of age, in the acute period of the disease - no later than 48 hours from the onset of the first symptoms.
For questions regarding literature, please contact the editor.
O. V. Kladova , Doctor of Medical Sciences T. F. Pogodina V. F. Uchaikin , Doctor of Medical Sciences, Professor, Academician of the Russian State Medical University, Moscow
Analogs
In the pharmacy chain there are drugs whose active ingredient is oseltamivir.
- "Nomides";
- "Influcein".
There are other drugs that differ in composition, but are similar in pharmacological action. The most popular of them:
- "Cycloferon";
- "Ergoferon";
- "Isoprinosine."
Elements of similar drugs have an immunotropic effect. The substances in their composition, acting as a single mechanism, suppress viral activity, improve well-being and accelerate the healing process.
Consumer Opinions
Most buyers are inclined to purchase cheaper analogues, but forget that inexpensive raw materials are used in their manufacture. Such drugs may cause more side symptoms with relatively low effectiveness. The effectiveness of Oseltamivir has been proven over time. It effectively fights severe forms of the disease. With its help, bird flu, swine flu and other strains of viruses are successfully treated.
Patients who took Oseltamivir testify that in the acute form they recovered faster than other patients. People whose treatment began in the first two days after infection did not have any complications.
Oseltamivir-Akrihin
Mechanism of action
Antiviral drug.
Oseltamivir is a prodrug, its active metabolite (oseltamivir carboxylate, OK) is an effective and selective inhibitor of neuraminidase of influenza viruses type A and B - an enzyme that catalyzes the process of release of newly formed viral particles from infected cells, their penetration into uninfected cells of the epithelium of the respiratory tract and further spread of the virus in the body.
Inhibits the growth of influenza virus in vitro
and suppresses the replication of the virus and its pathogenicity
in vivo
, reduces the release of influenza A and B viruses from the body. The concentration of OC required to inhibit neuraminidase by 50% (IC50) is 0.1-1.3 nM for influenza A virus and 2.6 nM for influenza B virus. The median IC50 value for influenza B virus is slightly higher and is 8.5 nM.
Clinical effectiveness
In studies conducted, oseltamivir had no effect on the formation of anti-influenza antibodies, including the production of antibodies in response to the administration of an inactivated influenza vaccine.
Natural Influenza Infection Research
In clinical studies of oseltamivir conducted during seasonal influenza infection, patients began receiving oseltamivir no later than 40 hours after the first symptoms of influenza infection appeared. 97% of patients were infected with influenza A virus and 3% of patients with influenza B virus.
Oseltamivir significantly shortened the period of clinical manifestations of influenza infection (by 32 hours). Patients with confirmed influenza who received oseltamivir had 38% less disease severity, expressed as the area under the curve for the total symptom index, compared with patients who received placebo. Moreover, in young patients without concomitant diseases, oseltamivir reduced by approximately 50% the incidence of influenza complications requiring the use of antibiotics (bronchitis, pneumonia, sinusitis, otitis media).
There was clear evidence of the drug's effectiveness in relation to secondary efficacy criteria related to antiviral activity: oseltamivir caused both a shortening of the time of viral shedding from the body and a decrease in the area under the viral titer-time curve.
Data obtained in a study on oseltamivir therapy in elderly and senile patients show that taking oseltamivir at a dose of 75 mg 2 times a day for 5 days was accompanied by a clinically significant decrease in the median period of clinical manifestations of influenza infection, similar to that in younger adult patients age, but the differences did not reach statistical significance.
In another study, influenza patients over 13 years of age who had concomitant chronic diseases of the cardiovascular and/or respiratory systems received oseltamivir in the same dosage regimen or placebo. There were no differences in the median period until clinical manifestations of influenza infection decreased in the oseltamivir and placebo groups, but the period of fever when taking oseltamivir was reduced by approximately 1 day. The proportion of patients shedding virus on days 2 and 4 became significantly smaller. The safety profile of oseltamivir in patients at risk did not differ from that in the general population of adult patients.
Treatment of influenza in children
In children aged 1-12 years (mean age 5.3 years) who had fever (≥37.8 °C) and one of the symptoms of the respiratory system (cough or rhinitis) during the period of circulation of the influenza virus among the population, we conducted double-blind, placebo-controlled study. 67% of patients were infected with influenza A virus and 33% of patients with influenza B virus.
The drug oseltamivir (when taken no later than 48 hours after the first symptoms of influenza infection appeared) significantly reduced the duration of the disease (by 35.8 hours) compared to placebo.
The duration of the disease was defined as the time until cough, nasal congestion, resolution of fever, and return to normal activity were resolved.
In the group of children receiving oseltamivir, the incidence of acute otitis media decreased by 40% compared to the placebo group. Recovery and return to normal activities occurred almost 2 days earlier in children receiving oseltamivir compared to the placebo group.
Another study involved children aged 6-12 years with asthma; 53.6% of patients had influenza infection confirmed by serology and/or culture. The median duration of disease in the group of patients receiving oseltamivir did not decrease significantly. But by the last 6 days of oseltamivir therapy, forced expiratory volume in 1 second (FEV1) increased by 10.8% compared with 4.7% in patients receiving placebo (p = 0.0148).
Preventing influenza in adults and adolescents
The prophylactic efficacy of oseltamivir against natural influenza A and B infections was demonstrated in 3 separate phase III clinical studies. While taking oseltamivir, about 1% of patients fell ill with the flu. Oseltamivir also significantly reduced the frequency of virus shedding and prevented transmission of the virus from one family member to another.
Adults and adolescents who were in contact with an ill family member started taking oseltamivir within two days of the onset of flu symptoms in family members and continued it for 7 days, which significantly reduced the incidence of influenza in contacts by 92%.
In unvaccinated and generally healthy adults aged 18–65 years, taking oseltamivir during an influenza epidemic significantly reduced the incidence of influenza (by 76%). Patients took the drug for 42 days.
In elderly and senile residents of nursing homes, 80% of whom were vaccinated before the season when the study was conducted, oseltamivir significantly reduced the incidence of influenza by 92%. In the same study, oseltamivir significantly (by 86%) reduced the incidence of influenza complications: bronchitis, pneumonia, sinusitis. Patients took the drug for 42 days.
Prevention of influenza in children
The preventive effectiveness of oseltamivir against natural influenza infection has been demonstrated in children from 1 to 12 years of age after contact with an ill family member or someone from their immediate environment. The main efficacy parameter was the incidence of laboratory-confirmed influenza infection.
In children who received oseltamivir (powder for oral suspension) at a dose of 30 to 75 mg once a day for 10 days and did not shed the virus at baseline, the incidence of laboratory-confirmed influenza decreased to 4% (2/47) compared with 21% (15/70) in the placebo group.
Preventing influenza in immunocompromised individuals
In immunocompromised individuals with seasonal influenza infection and in the absence of viral shedding initially, prophylactic use of oseltamivir led to a decrease in the frequency of laboratory-confirmed influenza infection accompanied by clinical symptoms to 0.4% (1/232) compared to 3% (7/231 ) in the placebo group. Laboratory-confirmed influenza infection, accompanied by clinical symptoms, was diagnosed in the presence of an oral temperature above 37.2°C, cough and/or acute rhinitis (all registered on the same day while taking the drug/placebo), as well as a positive return test result. Transcriptase polymerase chain reaction for influenza virus RNA.
Resistance
Clinical researches
In all patients carrying the OK-resistant virus, carriage was temporary, did not affect the elimination of the virus and did not cause a deterioration in the clinical condition.
| Patient Population | Patients with mutations leading to resistance | |
| Phenotyping* | Geno-and phenotyping* | |
| Adults and teenagers | 4/1245 (0,32%) | 5/1245 (0,4%) |
| Children (1-12 years old) | 19/464 (4,1%) | 25/464 (5,4%) |
*Complete genotyping was not performed in any of the studies.
When taking oseltamivir for the purpose of post-exposure prophylaxis (7 days), prophylaxis of family contacts (10 days) and seasonal prophylaxis (42 days) in persons with normal immune system function, no cases of drug resistance were observed.
In a 12-week seasonal prophylaxis study, no cases of resistance emerged in immunocompromised individuals.
Data from individual clinical cases and observational studies
In patients who did not receive oseltamivir, naturally occurring mutations of influenza A and B viruses were found that had reduced sensitivity to oseltamivir.
In 2008, the H275Y substitution mutation leading to resistance was found in more than 99% of 2008 H1N1 virus strains circulating in Europe. The 2009 H1N1 influenza virus (“swine flu”) was susceptible to oseltamivir in most cases.
Oseltamivir-resistant strains have been found in individuals with normal immune system function and in immunocompromised individuals taking oseltamivir. The degree of reduction in sensitivity to oseltamivir and the frequency of occurrence of similar viruses may vary depending on the season and region. Resistance to oseltamivir was found in patients with pandemic H1N1 influenza who received the drug for both treatment and prevention.
The incidence of resistance may be higher in younger patients and immunocompromised patients. Oseltamivir-resistant laboratory strains of influenza viruses and influenza viruses from patients treated with oseltamivir carry neuraminidase N1 and N2 mutations. Mutations leading to resistance are often neuraminidase subtype specific.
When deciding whether to use oseltamivir, the seasonal sensitivity of the influenza virus to the drug should be taken into account (the latest information can be found on the WHO website).
Preclinical data
Preclinical data based on standard pharmacological safety, genotoxicity and chronic toxicity studies do not indicate any particular hazard to humans.
Carcinogenicity
: Results from 3 carcinogenic potential studies (two 2-year studies in rats and mice for oseltamivir and one 6-month study in Tg:AC transgenic mice for the active metabolite) were negative.
Mutagenicity
: Standard genotoxic tests for oseltamivir and the active metabolite were negative.
Effect on fertility
: oseltamivir at a dose of 1500 mg/kg/day did not affect the generative function of male and female rats.
Teratogenicity
: in studies examining the teratogenicity of oseltamivir at doses up to 1500 mg/kg/day (in rats) and up to 500 mg/kg/day (in rabbits), no effect on embryofetal development was found. In studies examining antenatal and postnatal development in rats, an increase in the period of labor was observed when oseltamivir was administered at a dose of 1500 mg/kg/day: the safety limit between exposure in humans and the maximum non-effective dose in rats (500 mg/kg/day) for oseltamivir is 480 times higher, and for its active metabolite - 44 times higher. Exposure in the fetus was 15-20% of that in the mother.
Other
: Oseltamivir and the active metabolite are excreted into the milk of lactating rats. Limited data indicate that oseltamivir and its active metabolite are excreted in human breast milk. Based on the results of extrapolation of data obtained from animal studies, their amount in breast milk may be 0.01 mg/day and 0.3 mg/day, respectively.
In approximately 50% of tested guinea pigs, when administered maximum doses of the active substance oseltamivir, skin sensitization in the form of erythema was observed. Reversible eye irritation in rabbits has also been identified.
While very high single oral doses (657 mg/kg and above) of oseltamivir had no effect on adult rats, these doses were toxic to immature 7-day-old rat pups, including death. No undesirable effects were observed with chronic administration at a dose of 500 mg/kg/day from 7 to 21 days of the postnatal period.
Other reviews
- During a period of mass illness, the flu struck the entire family. On time, on the doctor’s recommendation, we began taking the antiviral drug Oseltamivir. No adverse reactions or serious complications were observed.
- After the temperature rose, I started taking Tamiflu. 5 days passed and the flu subsided. The condition improved and the symptoms no longer appeared. The treatment took place without side complications.
- The child was given a prescription by his pediatrician, but after the first dose he started vomiting. He felt sick. We were forced to change the drug.
Self-medication is a dangerous activity. Oseltamivir is an effective drug, but its effects are often accompanied by a negative reaction of the body to the substances, so the drug should be used only after approval by the doctor. It is known that Tamiflu does not treat ARVI.