What is Tanakan - description of the medicine
Tanakan is a drug based on herbal ingredients. The basis of its action is aimed at blood rheology, vasomotility of blood vessels and metabolic processes in tissues. The drug is designed to fill the brain tissue with oxygen and provide it with a sufficient amount of glucose. In addition, Tanakan normalizes the tone of blood vessels, arteries and veins, which improves blood microcirculation.
A course of taking the drug reduces the tendency to form blood clots and also prevents red blood cells from sticking together and breaking down. Tanakan protects against tissue hypoxia and prevents the appearance of free radicals, affects neurotransmitters, normalizing their catabolism, reuptake, release and ability to bind to membrane receptors.
Features of composition and pharmacological action
The main component of the medicine is an extract from the leaves of ginkgo biloba. Secondary ingredients are:
- milk sugar monohydrate;
- corn starch;
- colloidal silicon dioxide;
- microcrystalline cellulose;
- stearic acid;
- talc and starch.
The protective film consists of:
- from hypromellose;
- macrogol 6000 and 400;
- food additive E172;
- titanic anhydride.
The medicine may contain lemon or orange flavor, sodium saccharinate, 96% ethyl alcohol solution.
The medication has an angioprotective spectrum of action and is responsible for normalizing blood circulation in parts of the brain. The drug affects cellular metabolism, mechanical characteristics of blood (viscosity level, etc.), vasomotor reactions.
When taken, there is a sufficient supply of glucose and oxygen to the brain tissues, normalization of local blood circulation, and a change in the tone of blood vessels. The medicine reduces the risk of blood clots and prevents red blood cells from sticking together.
Tanakan stabilizes metabolic processes, prevents the formation of free radicals, and portable oxidation of fats in cell membranes. It has an antihypoxic spectrum of action, an effect on the release of processing and capture of neurotransmitters.
The maximum level of concentration of active components occurs after 1-2 hours, the half-life takes from 4 to 10 hours. The active ingredients are not subject to decay processes and are excreted in their original form with urine, small amounts - with feces.
Which is better: Tanakan or Nootropil
Nootropil, unlike Tanakan, is a completely synthetic drug. The mechanism of action of the two drugs is similar in terms of their effect on platelet formation, erythrocyte aggregation and the elasticity of vascular walls.
Side effects from using Nootropil are identical to the body’s negative responses to taking Tanakan. Considering the similarity of the mechanism of interaction on the hematopoietic system and blood vessels, it makes sense to replace one drug with another if an adverse reaction occurs during therapy. Due to the fact that the active ingredients of the drugs are different: in Tanakan these are extracts of ginkgo biloba leaves, and in Nootropil - piracetam, intolerance to piracetam may be a reason to switch to Tanakan.
Indications and contraindications
The medicine is recommended for patients:
- with reduced memory, mental performance and other functions, the list does not include dementia, metabolic problems, vascular abnormalities and Parkinson's syndrome;
- with extraneous noise in the ears;
- attacks of dizziness, intermittent claudication (as a consequence of chronic obliterating arteriopathy of the legs);
- coordination disorders – with vascular damage;
- disease and Raynaud's syndrome.
The list of contraindications for use is presented:
- exacerbation of gastric erosion;
- acute circulatory disorders in parts of the brain;
- reduced blood clotting rate;
- being a minor;
- pregnancy and breastfeeding;
- exacerbation of ulcers of the digestive system;
- acute form of myocardial infarction;
- allergy to component components.
The instructions prohibit therapy for certain pathological processes, including:
- congenital disorder of galactose metabolism;
- hypolactasia;
- lactase deficiency;
- glucose-galactose malabsorption.
Particular care in treatment is required for patients with alcohol dependence, diseases of the brain, liver, or after a TBI.
Why take Tanakan
The mechanism of action of the drug is based on the property of enriching cells with oxygen and improving blood fluidity, preventing red blood cell aggregation. Tanakan affects vasomotor reactions, tones blood vessels, inhibits platelet production, affects general metabolism, and has an antioxidant effect.
The medicine helps eliminate the consequences of traumatic brain injuries, improve brain performance, and slow down the development of Parkinson's disease and cognitive disorders.
The effect of the drug begins 1–1.5 hours after administration and persists for 4–8 hours. Tanakan’s components do not accumulate in the body, are completely filtered by the kidneys and excreted in the urine after 10 hours.
Are there any contraindications for Tanakan?
Like most angioprotectors, this drug is prohibited for use in the following conditions:
- internal bleeding;
- hemorrhagic stroke;
- exacerbations of stomach ulcers, erosive forms of gastritis;
- low blood clotting;
- myocardial infarction;
- chronic malabsorption of glucose, lactase deficiency;
- hypersensitivity to the components of the drug or an allergic reaction to them.
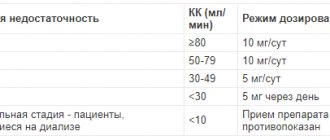
With simultaneous treatment with anticoagulants, taking aspirin and other antiplatelet agents, dosage adjustment and more careful monitoring of prothrombin time are required. The drug should be used with caution in cases of liver and kidney dysfunction.
Side effects of Tanakan
In most patients, the medicine does not cause unwanted reactions. Occasionally, during therapy, headaches, nervous agitation, worsening sleep, tachycardia, digestive disorders, and nausea are possible. Signs of an allergy to the drug may include swelling of the mucous membranes, itching, and urticaria.
The risk of side effects increases when combined with antibiotics, antimycotic drugs, antidepressants, and drugs to lower sugar levels.
In such cases, it is recommended to stop taking Tanakan and discuss the situation with your doctor.
Tanakan tablets p/o 40 mg No. 15x6
Name
Tanakan tablet 40 mg in blister pack No. 15x6
Description
The solution for oral administration is brownish-orange in color with a characteristic odor. Film-coated tablets are biconvex, round, brick-red in color. When broken, the tablets have a light brown color and a specific odor.
Main active ingredient
Ginkgo biloba leaf extract
Release form
15 tablets in a blister made of PVC/Aluminum foil. 2 or 6 blisters together with an insert are placed in a cardboard box.
Dosage
40 mg ml
special instructions
Before starting to use the drug, consult your doctor. Caution should be exercised when prescribing the drug simultaneously with other drugs whose metabolism is carried out through cytochrome P450-3A4 (CYP3A4). Since the drug contains lactose, it should not be prescribed to patients with congenital galactosemia, glucose or galactose malabsorption syndrome, or lactase deficiency. The use of the drug is contraindicated in children and adolescents under 18 years of age due to the lack of sufficient data. Patients with an increased risk of bleeding (hemorrhagic diathesis), as well as patients taking anticoagulant and antiplatelet drugs, should consult a doctor before taking the drug. Medicines containing ginkgo biloba may increase the risk of bleeding. The drug should be stopped 3-4 days before surgery. In patients with epilepsy, taking ginkgo biloba preparations can trigger the development of a seizure. The simultaneous use of ginkgo biloba and efavirenz is not recommended (see section “Interaction with other drugs”). If symptoms persist or worsen while taking the drug, consult a doctor. Interaction with other drugs and other forms of interactions The results of clinical trials of drug interactions of EGb761 with other drugs demonstrated either an increase or inhibition of the activity of cytochrome P450 isoenzymes. Thus, midazolam levels changed after administration of EGb 761, presumably due to their mutual effect on CYP3A4. Ginkgo biloba preparations should be used with caution with drugs that are metabolized through CYP3A4, as well as with drugs that have a narrow therapeutic index. Medicines based on ginkgo biloba, when taken simultaneously with anticoagulant drugs (for example, phenprocoumon, warfarin) or antiplatelet drugs (for example, clopidogrel, acetylsalicylic acid and other non-steroidal anti-inflammatory drugs) may cause a change in their action. Studies have not revealed the interaction of ginkgo biloba preparations with warfarin, however, when taking them together, it is recommended to carry out adequate monitoring of blood coagulation parameters at the beginning of treatment, when changing the dose, stopping treatment, or when replacing the ginkgo biloba preparation. Interaction studies with talinolol have shown that ginkgo biloba preparations may inhibit P-glycoprotein in the intestine. This may result in increased exposure to drugs that depend on P-glycoprotein for metabolism, such as dabigatran etexilate. Caution must be exercised when co-prescribing ginkgo biloba and dabigatran. Ginkgo biloba can increase the Cmax of nifedepine (in some individuals up to 100%), which is accompanied by dizziness and increased hot flashes. Concomitant use of ginkgo biloba and efavirenz is not recommended as plasma concentrations of efavirenz may be reduced due to induction of CYP3A4. If you are taking other medications at the same time, including those taken without a prescription, you should consult a doctor.
pharmachologic effect
Tanakan has a neuroprotective effect and prevents the formation of free radicals.
Pharmacodynamics
Improves the supply of oxygen and glucose to the brain, normalizes the tone of arteries and veins, improves microcirculation, helps improve blood flow, and prevents red blood cell aggregation; has an inhibitory effect on platelet activation factor; improves metabolic processes, has an antihypoxic effect on tissues; prevents the formation of free radicals and lipid peroxidation of cell membranes; affects the release, reuptake and catabolism of neurotransmitters (norepinephrine, acetylcholine) and their ability to bind to membrane receptors.
Indications for use
Encephalopathy of various origins with disorders of attention, memory, decreased intellectual abilities, sleep disturbances; disorders of peripheral circulation and microcirculation; neurosensory disorders of vascular origin - visual impairment, hearing impairment, coordination disorders; asthenic conditions.
Directions for use and doses
Tanakan is prescribed 120 mg per day (1 tablet or 1 ml solution 3 times a day), orally, with meals. For the treatment of asthenic disorders, the daily dose is 240 mg (2 tablets or 2 ml of solution 3 times a day). The duration of treatment with Tanakan is from 1 to 3 months. The tablets should be taken with half a glass of water, the oral solution should be dissolved in half a glass of water. When taking the drug in solution form, use the supplied dispensing pipette.
Use during pregnancy and lactation
Instructions for use Tanakan 30 pcs. tablets Composition and release form Oral solution - 100 ml: ginkgo biloba dry standardized extract (EGb 761) - 4000.00 mg (content of ginkgo heterosides - 24%; ginkgolide-bilobalides - 6%) excipients: sodium saccharinate; orange essence, soluble; lemon essence, soluble; ethanol (95%), purified water - up to 100 ml In dark glass bottles of 30 ml (complete with dosing pipette); 1 bottle in a cardboard pack. Film-coated tablets - 1 tablet: ginkgo biloba dry standardized extract (EGb 761) - 40.00 mg (content of ginkgo heterosides - 24%; ginkgolide-bilobalides - 6%) excipients: core: lactose monohydrate; MCC; corn starch; colloidal silicon dioxide; talc; magnesium stearate shell: hypromellose; macrogol 400; macrogol 6000; titanium dioxide; iron oxide red In contour cell packages of 15 pcs.; in a cardboard pack 2 or 6 packs. Description of the dosage form The solution for oral administration is brownish-orange in color with a characteristic odor. Film-coated tablets are biconvex, round, brick-red in color. When broken, the tablets have a light brown color and a specific odor. Characteristics A standardized and titrated preparation of plant origin, the effect of which is due to the influence on metabolic processes in cells, the rheological properties of blood, as well as the vasomotor reactions of blood vessels. Pharmacological action Tanakan has a neuroprotective effect and prevents the formation of free radicals. Pharmacodynamics Improves the supply of oxygen and glucose to the brain, normalizes the tone of arteries and veins, improves microcirculation, helps improve blood flow, prevents red blood cell aggregation; has an inhibitory effect on platelet activation factor; improves metabolic processes, has an antihypoxic effect on tissues; prevents the formation of free radicals and lipid peroxidation of cell membranes; affects the release, reuptake and catabolism of neurotransmitters (norepinephrine, acetylcholine) and their ability to bind to membrane receptors. Indications for use Encephalopathy of various origins with disorders of attention, memory, decreased intellectual abilities, sleep disturbances; disorders of peripheral circulation and microcirculation; neurosensory disorders of vascular origin - visual impairment, hearing impairment, coordination disorders; asthenic conditions. Contraindications for use: individual intolerance to Tanakan components; pregnancy and breastfeeding (no clinical data available). Use during pregnancy and children Contraindicated during pregnancy. Breastfeeding should be stopped during treatment.
Interaction with other drugs
Due to the fact that the drug in the form of a solution contains 0.45 g of 57% ethyl alcohol per dose (one dose), it is necessary to avoid simultaneous use with drugs of the following groups due to the possible occurrence of such reactions as hyperthermia, flushing of the skin, increased heart rate: cephalosporin antibiotics (cefamandole, cefoperazone, latamoxef); disulfiram; chloramphenicol; antidiabetic hypoglycemic agents (chlorpropamide, glibenclamide, glipizide, butamide); fungicides (griseofulvin); 5-nitroimidazoles (metronidazole, ornidazole, secnidazole, tinidazole), ketoconazole; cytostatics (procarbazine); tranquilizers.
Contraindications
individual intolerance to Tanakan components; pregnancy and breastfeeding (no clinical data available).
Compound
Oral solution - 100 ml: standardized dry ginkgo biloba extract (EGb 761) - 4000.00 mg (content of ginkgo heterosides - 24%; ginkgolide-bilobalides - 6%) excipients: sodium saccharinate; orange essence, soluble; lemon essence, soluble; ethanol (95%), purified water - up to 100 ml In dark glass bottles of 30 ml (complete with dosing pipette); 1 bottle in a cardboard pack. Film-coated tablets - 1 tablet: ginkgo biloba dry standardized extract (EGb 761) - 40.00 mg (content of ginkgo heterosides - 24%; ginkgolide-bilobalides - 6%) excipients: core: lactose monohydrate; MCC; corn starch; colloidal silicon dioxide; talc; magnesium stearate shell: hypromellose; macrogol 400; macrogol 6000; titanium dioxide; iron oxide red In contour cell packages of 15 pcs.; in a cardboard pack 2 or 6 packs. Description of the dosage form The solution for oral administration is brownish-orange in color with a characteristic odor. Film-coated tablets are biconvex, round, brick-red in color. When broken, the tablets have a light brown color and a specific odor.
Overdose
There are no known cases of drug overdose.
Storage conditions
3 years. Do not use after the expiration date stated on the packaging. Special precautions during storage Store at a temperature not exceeding 25°C. Keep out of the reach of children.
Adverse reactions and overdose
Sometimes during therapy the medication may have a non-standard effect:
- indigestion, attacks of nausea and vomiting;
- abdominal pain, diarrhea;
- swelling of the skin, obsessive itching;
- inflammatory processes in the dermis, hyperemia, rashes, eczema;
- nettle fever, shortness of breath, Quincke's edema;
- headaches with dizziness;
- fainting states.
If adverse reactions occur, you should stop taking the drug and consult your doctor. The abstract does not indicate known cases of drug overdose.