Description of the drug
Imovax Polio vaccine is an inactivated (non-live) vaccine for the prevention of polio.
Poliomyelitis is an acute infectious disease caused by RNA polioviruses (serovars I, II, III), characterized by damage to the central nervous system, lymphatic system, and gastrointestinal tract. The disease is characterized by the appearance of flaccid paralysis, mainly of the lower extremities. In the most severe cases, damage to the spinal cord leads to respiratory arrest and death. Clinically, poliomyelitis is manifested by fever, headaches and muscle pain, followed by the development of paralysis.
The primary goal of the polio vaccine is to eradicate the poliovirus.
The inactivated vaccine was developed to replace the live attenuated polio vaccine (oral polio vaccine - OPV) because OPV can cause a dangerous complication called vaccine-associated polio.
Vaccine-associated polio is a disease similar to polio, but the causative agent is not a virus, but a vaccine strain. The clinical manifestations and outcomes of the two variants of polio are similar. The introduction of at least two injections of the inactivated vaccine Imovax Polio reduces the likelihood of developing vaccine-associated polio to zero.
Vaccination of children with Infanrix and Imovax Polio vaccines in Varshavka
Polio
- an acute viral infection that affects the nervous system (gray matter of the spinal cord). Characterized by the appearance of flaccid paralysis, mainly of the lower extremities. In the most severe cases, damage to the spinal cord leads to respiratory arrest. The spread of the virus occurs through the patient’s secretions, and in some cases through airborne droplets. In 30% of cases, polio ends in disability, residual paralysis with muscle atrophy, in 10% of cases (with damage to the respiratory system) death occurs.
Whooping cough
– an infectious disease caused by the bacterium
Bordetella pertussis
. The most characteristic sign of whooping cough is a prolonged paroxysmal spasmodic cough, before the appearance of which it is difficult to distinguish whooping cough from other infections. Within one week, infected people manage to infect their surroundings. Infection occurs by airborne droplets.
Before the age of one year, whooping cough is severe and atypical, the frequency of deaths is high - in the pre-vaccination period, the mortality rate among children 1 year of age was 50-60%, for children of other ages - 8%. Every 10 children with whooping cough develop pneumonia, 3% of infants experience seizures, and 1% have brain damage (encephalopathy). Whooping cough and its complications can lead to the death of a child, with peak mortality occurring before the age of 1 year.
Diphtheria
- known since the time of Hippocrates under the name “deadly pharyngeal ulcer”, “suffocating disease”. During the epidemics of the first half of the 90s in the CIS countries, which arose due to low vaccination coverage, about 120 thousand people fell ill with diphtheria. Since 2002, Russia has again seen an increase in morbidity among adults, which is also caused by a decrease in revaccination coverage among the adult population.
The causative agent of diphtheria secretes a powerful toxin that destroys the membrane of nerves, damages red blood cells, and stops cellular respiration. The infection is transmitted by airborne droplets from sick and apparently healthy carriers (carriage can last up to 1 month, the number of carriers in the outbreak of the disease reaches 10%). It is possible to become infected with diphtheria through objects; transmission of the bacterium through food cannot be ruled out. Complications of diphtheria can be: myocarditis (inflammation of the heart muscle), polyneuritis (multiple nerve damage), paralysis, decreased vision, kidney damage.
Tetanus
– an infectious disease caused by the bacterium
Clostridium tetani
, affecting both humans and animals. The causative agent of the disease is widespread - in soil, animal and human feces. The route of infection in humans is through contaminated wounds of the skin and mucous membranes. The effect of tetanus toxin is expressed first in local, and subsequently in generalized spasms (tonic convulsions) of the muscles.
The prognosis of the disease is unfavorable. Death occurs as a result of spasm of the respiratory muscles and paralysis of the heart muscle. Despite emergency resuscitation, mortality rates reach 25% in developed countries, and 80% in developing countries. After an illness, immunity does not develop in people. Widespread vaccination has significantly reduced the incidence, but tetanus has not completely disappeared. In Russia, several dozen cases are registered annually among unvaccinated or incompletely vaccinated people.
Vaccination scheme
The course of primary vaccination with Imovax Polio consists of 3 injections with one dose of vaccine (0.5 ml) with an interval of at least 1.5 months, at the age of 3, 4.5 and 6 months, respectively.
Subsequent revaccinations are carried out with live polio vaccine within the periods specified by the National Calendar of Preventive Vaccinations of the Russian Federation: 18 months (or 1 year after the administration of the third vaccine) and 20 months (or 2 months after the first revaccination).
If there are medical contraindications to the use of a live vaccine, revaccinations can be carried out with inactivated polio vaccine according to the following scheme: the first revaccination of Imovax Polio is carried out 1 year after the third administration of the vaccine. Subsequent Imovax Polio revaccination is carried out every 5 years until the patient reaches the age of 18 years and then every 10 years.
Vaccine Imovax Polio (poliomyelitis)
Inactivated (killed) polio vaccine used for routine vaccination of children instead of OPV (live polio vaccine).
, France
Compound
: produced from 3 types of polio viruses, cultured on the VERO cell line and inactivated with formaldehyde.
Release form
: solution for injection, available in syringes or ampoules of 1 dose (0.5 ml).
Indicated for use
: prevention of polio, including for persons with contraindications to the use of “live polio vaccine”. Vaccination of pregnant women is possible.
Vaccination scheme
: The scheme for using the vaccine is determined by the “National Calendar of Preventive Vaccinations”. The Imovax Polio vaccine can be used in combination with other injectable forms of vaccines: for the prevention of diphtheria, tetanus, whooping cough, infections caused by Haemophilus influenzae type b, and hepatitis B (Infanrix, Euvax, ACT-Hib, Hiberix and others).
Contraindications
: allergy to streptomycin, acute infectious diseases accompanied by fever.
Advantages
: No risk of vaccine-associated paralytic poliomyelitis High immunogenicity, due to which fewer vaccinations are required (4 instead of 5 vaccinations before the child reaches 2 years of age). Greater effectiveness in the formation of general immunity, which plays a major role in individual protection against the virus. There is no risk of vaccination failure (dyspeptic symptoms, competition between vaccine polio- and enteroviruses). Thermal stability, possibility of storage under standard conditions Possibility of use in children with immunodeficiencies and those surrounded by people with this pathology. Immunity is acquired after the 3rd injection of the vaccine, strengthens with subsequent administrations of the drug and persists for at least 5 years after the first revaccination. Imovax Polio causes the production of a significant amount of antibodies neutralizing the polio virus, starting from the 2nd injection, regardless of the general condition of the immunized (immunodeficiency, intestinal pathology, dystrophy). After administration of 3 doses of the vaccine, immunity is observed in 95–100% of vaccinated people.
Application experience
: The Imovax Polio vaccine has been registered in Russia since 1996. Inactivated polio vaccines are recommended by WHO and the American Academy of Pediatrics (USA). The Imovax Polio vaccine has truly extensive experience in use abroad. Over the past 10 years, it has been used for routine vaccination in all developed European countries as a separate drug or as part of combination vaccines.
Compatibility of Isovax Polio with other vaccines
Imovax Polio can be used with an interval of 1 month or simultaneously (on the same day) with other vaccines (with the exception of the BCG vaccine - in accordance with the National Calendar of Preventive Vaccinations of the Russian Federation), provided that they are administered to different parts of the body using different syringes.
The use of the Imovax polio vaccine in combination with other vaccinations does not affect their immunogenicity (ability to develop immunity). Tolerability of vaccines does not deteriorate, and the number of adverse reactions does not increase. Administering several vaccines on the same day does not place an excessive burden on the immune system. Imovax polio can be used to continue and complete a vaccination course started with other polio vaccines, both live and inactivated. All vaccines in the Russian national vaccination calendar are interchangeable.
Imovax Polio® (vaccine for the prevention of polio, inactivated) (Imovax Polio)
Adverse events are listed according to systemic organ class and frequency of occurrence. The frequency was determined based on the following criteria: very common (≥ 1/10), common (≥ 1/100 to < 1/10), uncommon (≥ 1/1000 to < 1/100), rare (≥ 1/10000 to < 1/1(00), very rare <1/10000), frequency unknown (cannot be estimated from available data).
Clinical trial data
Local and general reactions
Very common: pain at the injection site, fever after vaccination with the 1st and 2nd dose;
Often: erythema at the injection site, fever after revaccination with the 3rd dose;
Uncommon: swelling at the injection site.
From very often to often: an increase in body temperature up to 38.5-39.5 ° C, transient within 24-48 hours after vaccination/re-vaccination with the drug Imovax Polio®.
Post-marketing surveillance data
Since reports of adverse events during commercial use of the drug were received very rarely and from a population with an unknown number of patients, their frequency was classified as “frequency unknown.”
The safety profile of Imovax Polio® vaccine does not differ significantly between patients of different ages, given the relative frequency of adverse events and the fact that some events are age specific (for example, seizures in infants and children from 2 to 11 years of age, myalgia/arthralgia in adolescents and adults). In addition, due to the simultaneous administration of other vaccines with the Imovax Polio® vaccine, a precise cause-and-effect relationship between the occurrence of adverse events and the use of the vaccine cannot be established.
The most common adverse events: local reactions and increased body temperature (approximately 20% and 10% of all registered adverse events, respectively).
Local and general reactions
Swelling, pain, redness at the injection site, appearing in the first 48 hours after injection and lasting 1-2 days; fever in the first 24-48 hours after vaccination.
From the central nervous system
Excitement, drowsiness, irritability in the first hours or days after vaccination (short-term).
From the nervous system
Brief convulsions, febrile convulsions in the first few days after vaccination; headache; transient mild paresthesia (mainly in the extremities) in the first 2 weeks after vaccination.
In very rare cases, seizures may occur later than the specified time. However, after 7 days there is no evidence linking seizures to vaccination.
From the skin and subcutaneous tissues
Rash, hives.
From the immune system
Allergic reaction, anaphylactic reaction, anaphylactic shock.
From the musculoskeletal system
Mild and transient arthralgia and myalgia in the first few days after vaccination.
From the hematopoietic organs
Lymphadenopathy.
In extremely premature infants (born at or before 28 weeks of gestation), prolonged breathing intervals may occur within 2-3 days after vaccination (see Precautions).
The patient should be warned that if he experiences any adverse events not listed in these instructions, he should consult a doctor.
How is vaccination carried out?
Vaccination is carried out in a vaccination room, in compliance with all sanitary requirements. All drugs are certified. A certificate for the drug is provided upon request.
Without reminders, before vaccination, the medical worker must show the drug and the expiration date of the vaccine.
Only sterile and disposable instruments are used. The vaccination must be carried out using disposable medical gloves.
On the day of vaccination, the child is examined by a pediatrician and the temperature is measured. In the absence of contraindications, vaccination is carried out. Information about the vaccination performed is entered into the card, vaccination certificate, and detailed recommendations for caring for the child in the post-vaccination period are given.
Before vaccination, the doctor will answer all your questions. Be sure to bring information about previous vaccinations to your appointment!
Please note that vaccination of a child, Mantoux test, Diaskintest can only be carried out in the presence of parents or legal representatives of the child (guardians), or if the accompanying person has a NOTARIZED power of attorney to carry out the manipulation (indicating the drug planned for administration) . Otherwise, vaccination will be denied. We comply with the laws of the Russian Federation.
Only here!
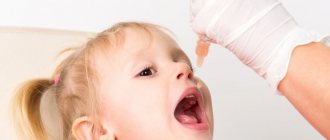
Oral polio vaccine "BiVac polio" bivalent, live attenuated, types 1.3
Attention! The vaccine is for oral use only.
The vaccine is used 4 drops per dose. The vaccination dose of the vaccine is instilled into the mouth with a dropper or pipette attached to the bottle 1 hour before meals. It is not allowed to take the vaccine with water or any other liquid, or to eat or drink within an hour after vaccination.
In accordance with the current edition of the National Preventive Vaccination Calendar, the first and second vaccinations against polio are given to children with the inactivated polio vaccine (IPV), in accordance with the instructions for the use of IPV.
The third vaccination and subsequent revaccinations against polio are given to children with the live oral polio prevention vaccine (LOP).
The first three vaccinations make up the vaccination course.
| Vaccinations | ||||||
| Vaccination | Revaccination | |||||
| IPV | PPV | PPV | ||||
| 1 | 2 | 3* | 4* | 5* | 6* | |
| Child's age | 3 months | 4.5 months | 6 months | 18 months | 20 months | 14 years |
* for children born to mothers with HIV infection, children with HIV infection, children in orphanages - the third vaccination and subsequent revaccinations against polio are carried out with IPV - a vaccine for the prevention of polio (inactivated).
For older children who have not received polio vaccinations in a timely manner, routine immunization is carried out according to the same scheme (first and second vaccinations - IPV, third vaccination and subsequent revaccinations - PPV).
In accordance with the current edition of the calendar of preventive vaccinations for epidemic indications, vaccination against polio for epidemic indications is carried out with an oral polio vaccine. When registering a case of polio caused by wild poliovirus, isolation of wild poliovirus in human biosamples or from environmental objects, mandatory vaccination is carried out for the following categories of citizens (contact persons in foci of polio, including those caused by wild poliovirus (or if the disease is suspected):
- children from 3 months to 18 years - once;
- medical workers - once;
- children arriving from polio-endemic countries (regions), from 3 months to 15 years - once (if there is reliable data on previous vaccinations) or three times (if they are absent);
- persons without a fixed place of residence (if identified) from 3 months to 15 years - once (if there is reliable data on previous vaccinations) or three times (if they are absent);
- persons in contact with arrivals from polio-endemic (polio-affected) countries (regions), from 3 months of life without age limit - once;
- persons working with live poliovirus, with materials infected (potentially infected) with wild poliovirus, without age limit, once upon hiring.
Features of the drug's action at the first dose
No specific effects of the drug were observed during the first dose. Actions of the doctor and the patient if one or more doses are missed. Extending the intervals between vaccinations is allowed in exceptional cases; in the presence of medical contraindications, shortening the intervals between the first three vaccinations is not allowed. It is allowed to reduce the interval between the third and fourth vaccinations to 3 months if the intervals between the first three vaccinations have been extended.
BiVac polio (poliomyelitis vaccine) oral solution 2 ml bottle N 10
International nonproprietary name
Vaccine to prevent polio
Dosage form
oral solution
Compound
1 dose (0.2 ml - 4 drops) contains:
Active component: polio virus, attenuated Sabin strains type 1 not less than 106.0 TCD50, type 3 not less than 105.5 TCD50 infectious units (IU) of the virus, expressed in tissue cytopathogenic doses (TCD50);
Excipients: magnesium chloride 0.018 g; kanamycin 30 mcg.
Description
Transparent liquid from yellowish-red to pink-raspberry color, without sediment, without visible foreign inclusions.
Pharmacotherapeutic group
MIBP - vaccine
ATX code
J07BF
Pharmacodynamics:
Immunological properties
The vaccine is a preparation from attenuated Sabin strains of polio virus 1 3 types grown on a primary culture of African green monkey kidney cells or on a primary culture of African green monkey kidney cells with one passage on a continuous cell culture of the Vero line and contains two types of virus (bivalent vaccine) in in the form of a solution with 05% lactalbumin hydrolyzate in Earle's solution. The vaccine creates lasting immunity to polio virus 13 types in (90-95)% of vaccinated people.
Indications:
Active prevention of polio.
Contraindications:
— Neurological disorders accompanying previous vaccination with oral polio vaccine;
— immunodeficiency state (primary) malignant neoplasms immunosuppression (vaccinations are carried out no earlier than 3 months after the end of the course of therapy);
- pregnancy;
- hypersensitivity to any component of the vaccine;
- severe reaction (temperature above 40 ° C) or complication to the previous administration of the drug;
- acute infectious or non-infectious diseases, exacerbation of chronic diseases - vaccinations are carried out 2-4 weeks after recovery or remission. For mild ARVI and acute intestinal diseases, vaccinations are carried out after the temperature has normalized.
Pregnancy and lactation:
Use during pregnancy is contraindicated.
The possibility and specifics of the medical use of the vaccine in women during breastfeeding have not been studied.
Directions for use and dosage:
Attention! The vaccine is for oral use only.
The vaccine is used 4 drops per dose. The vaccination dose of the vaccine is instilled into the mouth with a dropper or pipette attached to the bottle 1 hour before meals. It is not allowed to take the vaccine with water or any other liquid, or to eat or drink within an hour after vaccination.
In accordance with the current edition of the National Preventive Vaccination Calendar, the first and second vaccinations against polio are given to children with the inactivated polio vaccine (IPV) in accordance with the instructions for the use of IPV.
The third vaccination and subsequent revaccinations against polio are given to children with the live oral polio prevention vaccine (LOP).
The first three vaccinations make up the vaccination course.
| Vaccinations | ||||||
| Vaccination | Revaccination | |||||
| IPV | PPV | PPV | ||||
| 1 | 2 | 3* | 4* | 5* | 6* | |
| Child's age | 3 months | 45 months | 6 months | 18 months | 20 months | 14 years |
* children born from mothers with HIV infection, children with HIV infection, children staying in orphanages - the third vaccination and subsequent revaccinations against polio are carried out with IPV - a vaccine for the prevention of polio (inactivated).
For older children who have not received vaccination against polio within the prescribed period, routine immunization is carried out according to the same scheme (first and second vaccination - IPV, third vaccination and subsequent revaccinations - PPV).
In accordance with the current edition of the calendar of preventive vaccinations for epidemic indications, vaccination against polio for epidemic indications is carried out with an oral polio vaccine. When registering a case of poliomyelitis caused by wild poliovirus, isolation of wild poliovirus in human biosamples or from environmental objects, mandatory vaccination is carried out for the following categories of citizens (contact persons in foci of poliomyelitis, including those caused by wild poliovirus (or if the disease is suspected):
- children from 3 months to 18 years - once;
- medical workers - once;
- children arriving from polio-endemic (polio-affected) countries (regions) from 3 months to 15 years - once (if there is reliable data on previous vaccinations) or three times (if they are absent);
- persons without a fixed place of residence (if identified) from 3 months to 15 years - once (if there is reliable data on previous vaccinations) or three times (if they are absent);
- persons in contact with arrivals from polio-endemic (polio-affected) countries (regions) from 3 months of life without age limit - once;
— persons working with live poliovirus with materials infected (potentially infected) with wild polio virus without age limit, once upon hiring.
Features of the drug's action at the first dose
No specific effects of the drug were observed during the first dose.
Actions of the doctor and patient when one or more doses are missed
Lengthening the intervals between vaccinations is allowed in exceptional cases in the presence of medical contraindications; shortening the intervals between the first three vaccinations is not allowed.
It is allowed to reduce the interval between the third and fourth vaccinations to 3 months if the intervals between the first three vaccinations have been extended.
Side effects:
The following criteria were used to assess the incidence of adverse events: uncommon (≥ 1/1000 to <1/100) rare (≥ 1/10,000 to < 1/1000) very rare (< 1/10,000).
Reactions (except immediate allergic reactions in the first few hours after vaccination) usually cannot appear earlier than the 4th day and more than 30 days after administration of the vaccine.
Rarely - nonspecific symptoms: fever, vomiting, headache, not necessarily associated with taking the oral polio vaccine.
Very rarely - some vaccinated people may experience allergic reactions in the form of urticaria or Quincke's edema.
Isolated cases - both in vaccinated people and in people in contact with vaccinated people - have resulted in the occurrence of vaccine-associated paralytic poliomyelitis (VAPP). To prevent VAPP, the first two vaccinations against polio are carried out with the IPV vaccine.
Overdose:
An overdose does not lead to undesirable consequences.
Interaction:
Vaccinations against polio are allowed to be carried out on the same day with vaccination with DTP vaccine (ADS or ADS-M toxoid); simultaneous administration of polio vaccine with other drugs of the National Preventive Vaccination Calendar is allowed.
Immunosuppressants may reduce the immune response to oral polio vaccine by allowing vaccine viruses to multiply and prolonging the shedding of vaccine viruses in feces.
Special instructions:
All persons who are to receive preventive vaccinations must be examined by a doctor (paramedic).
In children's organized groups, it is necessary to plan vaccinations against polio for all children in the group at the same time.
Children who have not been vaccinated against polio should be separated from those recently vaccinated with PPV for a period of at least 60 calendar days from the date of vaccination.
To limit the circulation of the vaccine virus among those around the vaccinated child, the rules of personal hygiene of the child after vaccination should be observed (separate bed, potty, separate bedding, clothing from other children and the need to isolate the vaccinated child in the family from immunodeficiency patients).
In families where there are unvaccinated children due to age (newborns) or who have contraindications to polio vaccinations, the IPV vaccine should be used to immunize children belonging to the target groups.
All vaccinations against poliomyelitis are registered in established registration forms indicating the name of the drug, date of vaccination, dose, series number of reaction to the vaccine.
Unused vaccine from an opened vial can be stored for no more than 2 days at a temperature of 2 to 8 ° C in a vial tightly closed with a dropper or rubber stopper. The drug in a bottle with a damaged labeling, as well as with a change in its physical properties (transparency color, etc.), if the expiration date has expired, if the conditions of transportation and storage have been violated, is not suitable for use.
Precautions for use
The vaccine should not be administered parenterally!
If vomiting or diarrhea occurs during or immediately after administration of the vaccine, a second dose of the vaccine may be administered after these symptoms have resolved.
In the case of an upcoming planned operation, vaccinations should be carried out no later than 1 month before the operation. During emergency surgery, immunization should not be carried out earlier than 3-4 weeks after surgery.
Due to the potential risk of apnea in preterm infants (less than 28 weeks) and in children with a history of respiratory distress, continuous monitoring of respiratory activity is necessary due to the possibility of apnea occurring within 48-72 hours.
Impact on the ability to drive vehicles. Wed and fur.:
Does not affect the ability to drive vehicles and perform work requiring increased concentration and speed of psychomotor reactions.
Release form/dosage:
Oral solution 02 ml/dose.
Package:
20 ml (10 doses) in a bottle.
10 bottles along with instructions for use are placed in a cardboard pack.
Storage conditions:
In accordance with SP 3.3.2.2329-08 (amendments and additions to SP 3.3.2.1248-03), the vaccine is stored: at level 1 of the “cold chain” - at a temperature of minus 20 ° C and below when transporting the vaccine at a temperature range from 2 to 8 °C, subsequent re-freezing to minus 20 °C is allowed (at level 2 of the “cold chain”). At the 3rd and 4th levels of the “cold chain” the vaccine is stored at a temperature of 2 to 8 °C.
Keep out of the reach of children.
Transportation conditions
In accordance with SP 3.3.2.2329-08 at temperatures from 2 to 8 °C.
Best before date:
2 years at temperatures minus 20 °C and below
6 months at temperatures from 2 to 8 °C.
Vacation conditions
On prescription