Pharmacological properties of the drug Meloxicam-ratiopharm
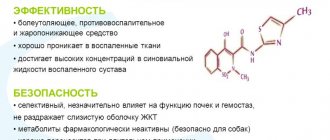
Meloxicam is an NSAID of the oxicam group with anti-inflammatory, analgesic and antipyretic properties that are associated with selective inhibition of the COX-2 isoenzyme. The selectivity coefficient IC50 for meloxicam is 2. Meloxicam is almost completely absorbed from the gastrointestinal tract. Absolute bioavailability after oral administration is about 89%. After a single oral dose, the maximum concentration in the blood plasma is reached after 5–6 hours. With repeated administration, an equilibrium state is achieved after 3–5 days from the start of use. When taken orally at a dose of 7.5 or 15 mg of meloxicam 1 time per day, the concentration in the blood plasma (Cmin–Cmax) at steady state reaches 0.4–1.0 mg/l (7.5 mg) or 0.8– 2.0 mg/l (15 mg), respectively. With long-term use, the concentration in the blood plasma at steady state does not change. Absorption does not change when taken simultaneously with food. The bioavailability of meloxicam after intramuscular administration is 89%. The maximum concentration in the blood is achieved 1 hour after administration. Meloxicam has a high degree of binding to blood proteins, mainly albumin (99%). Meloxicam enters the synovial fluid, the concentration in it is equal to the concentration in the blood plasma. The volume of distribution is on average 11 liters. Individual variations are approximately 30–40%. Meloxicam is metabolized by liver enzymes. Four different pharmacologically inactive metabolites of meloxicam have been identified in urine. The main metabolite, 5'-carboxymeloxicam (60%), is formed by oxidation of intermediate metabolites 5'-hydroxymethylmeloxicam. The amount of 5'-hydroxymethylmeloxicam released unchanged is 9%. In vitro , it was found that the initial stage of this transformation is carried out mainly through CYP 2C9 with minor participation of CYP 3A4. The formation of two other metabolites (16 and 4% of the dose taken, respectively) is associated with the action of peroxidase. Meloxicam is excreted mainly in the form of metabolites, in equal parts with urine and feces. Meloxicam is detected in urine in insignificant (trace) quantities. The average half-life is about 20 hours. The total plasma clearance averages 8 ml/min. When administered orally in therapeutic doses (7.5 and 15 mg), meloxicam exhibits linear pharmacokinetics.
Meloxicam tablets: main characteristics
Meloxicam is a non-steroidal drug that has anti-inflammatory and antipyretic effects. It helps in eliminating pain and works as an analgesic. Available in 3 forms:
- Pills.
- Solution (in glass ampoules).
- Suppositories.
The active ingredient is meloxicam, a derivative of enolic acid. Dispensed only with a doctor's prescription. The drug is stored at room temperature (no more than 25 degrees) within the general shelf life (no more than 2 years).
Use of the drug Meloxicam-ratiopharm
The solution for injection is administered only intramuscularly (iv administration is contraindicated). It is advisable to prescribe parenteral administration only during the first days of treatment. For further therapy, it is recommended to use tablets. Arthrosis in the acute phase: dose 7.5 mg/day. If the condition does not improve, you can increase to 15 mg/day. Rheumatoid arthritis, ankylosing spondylitis: dose 15 mg/day. Depending on the therapeutic effect, the dose can be reduced to 7.5 mg/day. The daily dose of meloxicam should not exceed 15 mg. Take the daily dose 1 time during meals with a glass of water or other liquid. Special groups of patients Elderly patients with an increased risk of side effects In elderly patients, the recommended dose for long-term therapy of rheumatoid arthritis or ankylosing spondylitis is 7.5 mg / day. In patients with an increased risk of side effects, therapy should be started with a dose of 7.5 mg/day. Impaired renal function In dialysis patients with severe renal impairment, the daily dose of meloxicam should not exceed 7.5 mg. In patients with mild or moderate renal impairment (creatinine clearance more than 25 ml/min), there is no need to reduce the dose. Impaired liver function In patients with mild or moderately severe liver dysfunction, there is no need to reduce the dose of the drug.
Buy Meloxicam tablets 15 mg No. 20 in pharmacies
Instructions for use Meloxicam
Buy Meloxicam tablets at the pharmacy. 15 mg No. 20 Dosage forms tablets 15 mg Synonyms Amelotex Arthrozan Bi-xicam Melox MELOXICAM AVEXIMA Meloflex Rompharm Mesipol Mirlox Movalis Movasin Oxycamox Group Anti-inflammatory drugs - oxicams International nonproprietary name Meloxicam Composition Active ingredient - meloxicam. Manufacturers Kanonpharma Production (Russia) Pharmacological action Has anti-inflammatory, analgesic, antipyretic effects. Well absorbed. Passes through histohematic barriers and penetrates into the synovial fluid. Subjects to biotransformation with the formation of inactive metabolites. Excreted in feces and urine in equal proportions. Side effects From the gastrointestinal tract: dyspepsia, nausea, vomiting, abdominal pain, esophagitis, gastroduodenal ulcers, constipation or diarrhea, flatulence. From the nervous system and sensory organs: dizziness, headache, ringing in the ears, drowsiness. Other: edema, increased blood pressure, palpitations, increased creatinine and/or serum urea, anemia, leukopenia, thrombocytopenia, photosensitivity, allergic reactions (rash, itching, bronchospasm, urticaria). Indications for use Rheumatoid arthritis, osteoarthritis, arthrosis and other inflammatory and degenerative diseases of the joints, accompanied by pain. Contraindications Hypersensitivity (including to NSAIDs of other groups), gastric ulcer in the acute stage, severe liver or end-stage renal failure, pregnancy, breastfeeding, adolescence (up to 15 years). Overdose Symptoms: increased side effects. Treatment: gastric lavage and symptomatic therapy, administration of cholestyramine to accelerate elimination. Interaction Increases the risk of bleeding against the background of indirect anticoagulants, ticlopidine, heparin, thrombolytics. Increases the concentration of lithium in the blood plasma. Increases the toxicity of methotrexate (cytopenia), cyclosporine (nephrotoxicity). Reduces the effectiveness of antihypertensive drugs. Special instructions Use with caution for diseases of the upper gastrointestinal tract in the anamnesis, in combination with anticoagulants, myelotoxic drugs, incl. methotrexate. Prescribing NSAIDs to patients with reduced renal blood flow and volume may accelerate renal decompensation. The risk of developing such reactions is especially high in patients with symptoms of dehydration, congestive heart failure, liver cirrhosis, nephrotic syndrome and severe kidney disease, in patients receiving diuretics, as well as in those who have undergone significant surgery leading to hypovolemia (careful monitoring is required from the start of treatment diuresis and kidney function). If there is a significant deviation from the norm in the level of serum transaminases and other indicators characterizing liver function, you should stop taking it and conduct control laboratory tests. Prescribe with caution to elderly, weakened and exhausted patients.
Method of administration and dosage The drug is taken orally with meals once a day. Recommended dosage regimen. Rheumatoid arthritis: 15 mg per day. After achieving a therapeutic effect, the dose can be reduced to 7.5 mg per day. Osteoarthritis: 7.5 mg per day. If necessary, the dose can be increased to 15 mg per day. Ankylosing spondylitis: 15 mg per day. The maximum daily dose should not exceed 15 mg. In patients with an increased risk of side effects, as well as in patients with severe renal failure on hemodialysis, the dose should not exceed 7.5 mg per day. Pregnancy and lactation. The drug is not recommended for use during pregnancy and lactation. Storage conditions List B. In a dry place, protected from light, at room temperature.
Contraindications to the use of the drug Meloxicam-ratiopharm
Hypersensitivity to meloxicam or other NSAIDs, including acetylsalicylic acid (contraindicated in patients who experience symptoms of asthma, nasal polyps, angioedema or urticaria after taking acetylsalicylic acid or other NSAIDs); During pregnancy and breastfeeding; gastrointestinal ulcers (both in history and in the acute phase); severe liver or kidney failure; gastrointestinal and cerebrovascular bleeding or bleeding of other localization; severe uncorrectable heart failure; age up to 15 years.
Side effects of the drug Meloxicam-ratiopharm
When assessing the frequency of side effects, the following statistics are used as a basis:
Often | ≥1/10 patients |
often | ≤1/10 but ≥1/100 patients |
Sometimes | ≤1/100 but ≥1/1000 patients |
rarely | ≤1/1000 but ≥1/10,000 patients |
very rarely | ≤1/10,000 patients |
From the peripheral blood: often - anemia; sometimes - changes in the blood picture - thrombocytopenia, leukopenia, agranulocytosis. From the immune system: rarely - allergic reactions. From the side of the central nervous system: often - loss of consciousness, headache; sometimes - dizziness, tinnitus; rarely - mood swings, drowsiness and nightmares, confusion. From the side of the organ of vision: rarely - impaired visual acuity. From the cardiovascular system: sometimes - tachycardia, increased blood pressure, skin flushing with a feeling of heat. From the respiratory system: rarely - patients with a history of allergic reactions to acetylsalicylic acid or other NSAIDs may experience asthma attacks. From the gastrointestinal tract: often - pain in the epigastric region, nausea and vomiting, gastralgia, flatulence, constipation or diarrhea; sometimes - gastrointestinal bleeding, gastric ulcer, esophagitis, stomatitis; rarely - gastrointestinal perforation, gastritis, colitis. In elderly patients, peptic ulcers, perforations, or gastrointestinal bleeding may be particularly severe. From the liver and biliary tract: sometimes - impaired liver function; rarely - hepatitis. From the skin: often - itching, skin rash; sometimes - urticaria; rarely - Stevens-Johnson syndrome, toxic epidermal necrolysis/Lyell's syndrome, angioedema of the skin and/or mucous membrane, erythema multiforme, photosensitivity. From the urinary system: sometimes - renal dysfunction (increased concentrations of urea and creatinine in the blood serum); rarely - renal failure. General disorders: often - swelling.
Side effects
When using Meloxicam in different forms, the following side effects may be observed:
- allergies (itching, rash, blistering);
- anemia, leukopenia;
- headache, dizziness;
- high drowsiness;
- swelling;
- increased urea content;
- increased heart rate;
- rush of blood to the chest, neck and face;
- increase in pressure.
In rare cases, other side effects were observed: nephrotic syndrome, kidney necrosis, and tinnitus.
Special instructions for the use of Meloxicam-ratiopharm
Given the risks associated with the use of meloxicam, the duration of its use should be as short as possible, and the daily dose should be the minimum effective. Before starting treatment with meloxicam, it is necessary to undergo a course of treatment for esophagitis, gastritis, peptic ulcers of the stomach and duodenum (such patients may have a history of relapses during treatment with meloxicam). In patients with a history of gastrointestinal pathology, it is necessary to assess the condition of the digestive tract in order to detect gastrointestinal bleeding. As with the use of other NSAIDs, asymptomatic gastrointestinal bleeding or perforation, sometimes resulting in death, has been reported during treatment with meloxicam. In elderly people, gastrointestinal bleeding or gastrointestinal perforation is more severe. In such cases, treatment with meloxicam is discontinued. Severe life-threatening allergic reactions (such as anaphylactic reactions) may occur when using NSAIDs, including meloxicam. In this case, the use of meloxicam should be stopped immediately and appropriate treatment should be carried out. In isolated cases, NSAIDs can cause interstitial nephritis, glomerulonephritis, necrosis of the hepatic papillae, or nephrotic syndrome. A transient increase in liver function tests is noted. In most cases, these disorders are temporary. In case of persistent violation of these indicators, the use of meloxicam should be discontinued. By causing potassium, sodium and water retention, as well as interacting with diuretics, NSAIDs can worsen the condition of patients with heart failure or hypertension (arterial hypertension). The use of NSAIDs may cause decompensation of latent renal failure. Kidney function returns to normal after discontinuation of therapy. Use with caution in the elderly, with heart failure, liver cirrhosis or renal failure, as well as in patients using diuretics. When treating such patients, it is necessary to monitor diuresis and renal function. The effect of treatment should be regularly monitored, assessing the need for its continuation. Like other drugs that inhibit COX or prostaglandin synthesis, meloxicam may mask the symptoms of infectious diseases (such as fever). In some cases, the drug has a negative effect on reproductive function, so it is not recommended for women planning pregnancy. The tablets contain lactose, so the drug should not be prescribed to patients with hereditary lactose deficiency, galactosemia or glucose/galactose malabsorption syndrome. There is no data on the negative effect of meloxicam on the ability to drive vehicles or operate machinery. If there are disorders of the central nervous system (decreased visual acuity, increased fatigue, dizziness or other disorders), these types of activities are contraindicated.
Drug interactions Meloxicam-ratiopharm
Pharmacodynamics: Concomitant use of several NSAIDs (including salicylates) may increase the risk of developing erosive and ulcerative lesions of the gastrointestinal tract due to a synergistic effect, therefore the use of meloxicam with other NSAIDs is not recommended. When using meloxicam with diuretics, the patient must drink sufficient fluids, and regular medical monitoring of renal function is required before and during treatment. Oral anticoagulants: combined use increases the risk of bleeding due to inhibition of platelet function and damage to the mucous membrane of the stomach and intestines. Therefore, combined use with NSAIDs and oral anticoagulants is not recommended. Thrombolytic and antithrombotic drugs: when used in combination with meloxicam, an increased risk of bleeding is possible (periodic monitoring of blood coagulation parameters is necessary). ACE inhibitors and other antihypertensive drugs: simultaneous use in elderly patients with symptoms of dehydration may provoke the development of acute renal failure. In addition, combined use with meloxicam may reduce their hypotensive effect. Cyclosporine: the nephrotoxic effect of cyclosporine is enhanced. Contraception: The effectiveness of birth control may be reduced. Pharmacokinetic Lithium: NSAIDs may increase serum lithium concentrations to toxic levels (decreased renal excretion of lithium). Therefore, the simultaneous use of NSAIDs with lithium preparations is not recommended. If their combined use is necessary, the level of lithium in the blood serum should be carefully monitored before, during and after the end of the course of therapy with meloxicam and lithium preparations. Methotrexate: increases the negative effect on the blood system (threat of anemia and leukopenia). Periodic monitoring of the hemogram is necessary. Cholestyramine accelerates the elimination of meloxicam, increasing the clearance of meloxicam by 50%, reducing its half-life by 13±3 hours. This interaction is of clinical significance. When taken simultaneously with antacids, cimetidine and digoxin, no significant clinical interaction is observed. NSAIDs reduce the effectiveness of contraceptive intrauterine devices.
Meloxicam - a broad view of the problem of use
Table 1. Associations between acute renal failure and NSAID use
Nonsteroidal anti-inflammatory drugs (NSAIDs) are a class of drugs that perhaps no doctor can do without. Diseases of the musculoskeletal system, inflammatory and traumatic lesions are the range of diseases in the treatment of which NSAIDs are used, because they have an analgesic and anti-inflammatory effect.
NSAIDs, according to international and national guidelines, are prescribed either as a first-choice analgesic or as a second-choice analgesic when paracetamol fails to provide sufficient pain relief for various musculoskeletal complaints such as back pain, shoulder pain, osteoarthritis [1–3].
The use of traditional non-selective NSAIDs is known to be associated with frequent adverse events from the gastrointestinal tract (GIT), primarily in its upper parts [4]. The need to reduce the number of such complications has led to the development of new classes of NSAIDs, mainly selective cyclooxygenase (COX)-2 inhibitors and specific (highly selective) COX-2 inhibitors [5].
According to the mechanism of action, all existing NSAIDs can be divided into four groups (and the division into “predominant” and “specific” COX-2 inhibitors is largely arbitrary) [6]:
selective COX-1 inhibitors (low doses of acetylsalicylic acid);
non-selective COX inhibitors (most “standard” NSAIDs);
predominantly selective COX-2 inhibitors (nimesulide, meloxicam);
specific (highly selective) COX-2 inhibitors (coxibs).
The last two groups of NSAIDs were developed in connection with the assumption that the anti-inflammatory, analgesic and antipyretic effects of NSAIDs are due to inhibition of COX-2, and the most common side effects are associated with inhibition of COX-1 activity. This became the basis for the synthesis of new NSAIDs - selective COX-2 inhibitors (nimesulide, meloxicam), and then even more selective, specific COX-2 inhibitors (coxibs).
The desire to achieve selectivity regarding COX-2 was dictated primarily by the desire to obtain drugs that are no less effective than “standard” NSAIDs, but less dangerous in terms of undesirable effects, primarily in terms of their effect on the gastrointestinal mucosa. The comparable therapeutic efficacy of COX-2 selective and traditional NSAIDs has been repeatedly confirmed in animal and clinical studies [7].
The selectivity of NSAIDs for COX inhibition is quite conditional and relative. This is because selective COX-2 inhibitors have a high degree of blockade of COX-2, while at the same time they slightly block COX-1. In addition, the selectivity of COX inhibition may be dose dependent and vary depending on the assay used to assess COX activity. For example, in the United States, etodolac and meloxicam are considered “conventional” (i.e., non-COX-2 specific) NSAIDs. In Canada and most European countries, meloxicam in particular is considered a COX-2 selective NSAID based on William Harvey's modified whole blood assessment methods [8].
Low back pain (LBP) is a very common clinical syndrome. It is estimated that approximately 84% of adults may experience at least one episode of LBP during their lifetime, and relapses occur in 5–60% of patients [9].
LBP is the most common condition for which patients seek medical help [10]. In the United States, back pain, which is associated with lost work time among employees aged 40 to 65 years, is estimated by employers at $7.4 billion per year [11], and, according to some estimates, $50 billion is spent annually on the treatment of LBP [ 12]. In Australia, direct medical costs associated with the treatment of LBP are estimated to be more than $1 billion per year, with an additional $8 billion in indirect costs [13]. Although the treatment prognosis for most patients with the first attack of LBP is positive, in 20% of cases it is possible to transform LBP into chronic LBP, which lasts 3 months or more [14]. Three-quarters of the total direct and indirect health care costs and lost productivity associated with LBP are attributable to chronic pain [15]. Therefore, it is important to effectively treat acute LBP to prevent it from developing into a chronic form.
A systemic analysis using the Cochrane database of the use of NSAIDs in LBP revealed the same effectiveness of various NSAIDs, including selective COX-2 inhibitors, but with the best tolerability of the latter [16], and therefore this group of drugs can be recommended to a patient with LBP - for example, the drug Liberum (meloxicam), which is available in the form for intramuscular injection 15 mg in an ampoule. The advantages of the drug include the fact that it is produced in accordance with GMP standards, as well as the optimal price/quality ratio. Liberum belongs to the class of oxicams (enolic acid derivative). The drug predominantly selectively inhibits the enzymatic activity of COX-2. Meloxicam is structurally different from other COX-2 inhibitors, such as coxibs, and binds to the top of the COX-2 channel, rather than to the side of this enzyme, like celecoxib [17]. Liberum does not have a damaging effect on the gastrointestinal tract, cardiovascular system, or kidneys.
For example, acute renal failure (ARF) may develop. A thorough analysis of the risk of developing acute renal failure in elderly patients was carried out in the USA [18]. According to the program for assessing the effectiveness and safety of drugs prescribed between 1999 and 2004. in persons over 65 years of age, the side effects of NSAIDs were assessed when taken for 6 months or more. Patients receiving two NSAIDs simultaneously were excluded from the study. Of 183,446 patients with a mean age of 78 years, AKI leading to hospitalization occurred in 870 patients. The most common NSAID prescribed to this group of patients was celecoxib, which was taken by every third patient. Table 1 shows the relative risk and 95% confidence interval of developing AKI when taking various NSAIDs in comparison with celecoxib.
A significant increase in the risk of developing acute renal failure by 50% and 100% was obtained for ibuprofen and indomethacin, respectively. The table shows that meloxicam has the lowest risk of developing acute renal failure among the selective and non-selective NSAIDs analyzed, i.e. Liberum has a better tolerability profile compared to non-selective NSAIDs (for example, diclofenac) due to the selective suppression of COX-2.
The pharmacokinetic features include the following: binding to plasma proteins is 99%. Passes through histohematic barriers and penetrates into the synovial fluid. Concentration in synovial fluid reaches 50% of Cmax in plasma. It is excreted equally in feces and urine, mainly in the form of metabolites. 1/2 of meloxicam is excreted unchanged through the intestines in 15–20 hours. Plasma clearance averages 8 ml/min.
In case of LBP, NSAIDs and B vitamins are often combined, since neurotropic vitamins enhance the analgesic effectiveness of NSAIDs and themselves have analgesic activity. Taking this into account, a combination of Vitaxon and Liberum can be proposed. Vitaxon contains thiamine hydrochloride 100 mg, pyridoxine hydrochloride 100 mg, cyanocobalamin 1 mg and lidocaine hydrochloride 20 mg, release form - 2 ml in an ampoule. By combining Liberum and Vitaxon, it is possible to reduce the duration of treatment, reduce the dosage of NSAIDs, and increase patient compliance. With such synergy, complex and safe effects of these two drugs, this combination can be recommended in the practice of both neurologists and therapists. Liberum is prescribed 1 ampoule IM 1 time per day, the course of treatment is usually 3–5 days, Vitaxon is prescribed in parallel 1 ampoule IM 1 time per day daily for 5–10 days, subsequently switching to more infrequent injections (2-3 times a week for 2-3 weeks).
It is interesting to note that the parenteral form of meloxicam for intramuscular administration has its own characteristics. Thus, due to the significant half-life of meloxicam, its concentration when taking the tablet form stabilizes in the patient’s blood only on the 3rd–4th day. Therefore, to quickly relieve severe or acute pain, it is necessary to use a parenteral form, which is also important in the treatment of acute LBP. Pharmacokinetic studies have shown that intramuscular use of meloxicam leads to faster absorption of the drug than its oral administration; maximum plasma concentration is achieved within 1.5 hours after IM administration compared to 5–7 hours after oral administration [19]. In this case, 90% of Cmax is achieved within 30–50 minutes after injection. This increase in absorption determines the faster onset of action of meloxicam administered intramuscularly compared to oral administration.
In order for IM administration to be considered as an alternative to the oral route of administration, very good local tolerability is required. However, many NSAIDs are poorly tolerated when administered intramuscularly, causing local tissue irritation and necrosis, often in combination with systemic adverse events [20]. Studies in animal models (rabbits) have shown that the local tolerability of meloxicam is better than that of other NSAIDs. After its intramuscular administration, no histopathological changes were detected, while when using piroxicam or diclofenac, a large area of necrosis developed. In addition, if we talk about the drug Liberum, it has a very convenient packaging - 5 ampoules per package, which is usually enough for acute pain syndrome to regress.
The effectiveness of meloxicam in the form for intramuscular administration and for oral administration at a dose of 15 mg was compared in 113 patients with acute sciatica. It was found that both dosage forms of the drug significantly reduced pain. The average time of onset of the analgesic effect did not differ significantly in patients receiving meloxicam intramuscularly or orally and was 80 and 89 minutes. In both treatment groups, the severity of spontaneous pain significantly decreased (compared to the initial level); no significant differences were found. But IM administration of meloxicam was superior to oral administration of the drug in terms of maximum reduction in induced pain, assessed when raising a straight leg (p
Another study examined the effectiveness of a single IV dose of meloxicam 15 mg and IM diclofenac 75 mg followed by 7 days of oral meloxicam 15 mg and diclofenac 100 mg, respectively, in 183 patients with acute lumbago. IV meloxicam demonstrated a significantly faster mean time to analgesic effect (30 min) compared with diclofenac (60 min). The reduction in pain within 30 minutes after injection was also statistically significantly higher in the meloxicam group (p=0.048). Overall efficacy scores in the meloxicam group were significantly better than in the diclofenac group, both as assessed by investigators (p=0.02) and by patients (p=0.01). In addition, in the meloxicam group, as assessed by investigators and patients, general and local tolerability was significantly higher (p
Osteoarthritis (OA) is a disease that is usually classified as degenerative joint disease, characterized by the development of synovitis and is an indication for the use of drugs that relieve the inflammatory process.
It has been shown that the effectiveness of meloxicam in the treatment of patients with OA is equal to the effectiveness of non-selective NSAIDs (diclofenac, piroxicam) [23], and tolerability is much better [24].
The drug has demonstrated equivalent effectiveness to that of non-selective NSAIDs in other rheumatological diseases: rheumatoid arthritis, ankylosing spondylitis [25].
A systematic review was conducted of the clinical effectiveness and cost-effectiveness of selective COX-2 inhibitor NSAIDs (etodolac, meloxicam, celecoxib, rofecoxib, valdecoxib and lumiracoxib) compared with non-selective NSAIDs in the treatment of OA and rheumatoid arthritis [26]. Results from 16 randomized controlled trials that compared meloxicam with placebo or non-selective NSAIDs (naproxen, diclofenac, nabumetone or piroxicam) indicate that there is a low risk of adverse effects with meloxicam at a daily dose of 7.5–22.5 mg. sides of the gastrointestinal tract.
Diabetic retinopathy (DR) is the most common cause of blindness in people over 50 years of age. There is a body of evidence suggesting that DR is an inflammatory disease. Animal models with DR show that at the onset of the disease, vascular permeability increases, leukostasis appears, resulting in the formation of vascular dysfunction and the death of the capillary endothelium. Therefore, a number of anti-inflammatory drugs, such as etanercept, aspirin or meloxicam, reduce the level of leukostasis and reduce the possibility of death of the endothelium of the retinal capillaries [27].
Thus, based on the results of randomized clinical trials and post-registration studies of meloxicam, it can be stated that the drug [28]:
clear analgesic and anti-inflammatory activity was revealed in chronic diseases of the joints and spine, as well as in acute pain syndromes (lumboischialgia);
high gastrointestinal tolerance has been confirmed;
large-scale pharmacoepidemiological studies confirm the low risk of severe side effects from the gastrointestinal tract, previously established in controlled clinical trials and in the process of meta-analysis;
no increase in the incidence of cardiovascular toxicity was noted.
Therefore, meloxicam, and in particular Liberum, can be recommended for widespread use in patients with various diseases that are accompanied by pain syndromes.
Meloxicam-ratiopharm overdose, symptoms and treatment
Symptoms of acute NSAID overdose are often accompanied by drowsiness, nausea, vomiting and gastralgia, which usually resolve with symptomatic therapy. Gastrointestinal bleeding may also occur. Severe poisoning can lead to increased blood pressure, acute renal failure, liver dysfunction, respiratory depression, coma, convulsions, collapse and cardiac arrest. Anaphylactoid reactions have been reported during therapeutic doses of NSAIDs in cases of overdose. After an overdose of NSAIDs, patients are given supportive and symptomatic therapy in accordance with the severity of the overdose and intoxication. Clinical studies have found that three doses of 4 g of cholestyramine orally accelerates the elimination of meloxicam.
Overdose and interaction with other drugs
If the dosage is violated, the side effects described above are observed. In this case, gastric lavage is indicated. An antidote and antagonist for Meloxicam has not been developed.
If taken together with myelotoxic medications, this may lead to increased hematotoxicity. It is not advisable to take it together with non-steroidal anti-inflammatory drugs - this can lead to ulcerative lesions and bleeding in the digestive organs.
When taken together with anticoagulants and blood pressure lowering agents, the risk of bleeding increases. When taken together with Cyclosporine, toxic effects on the kidneys are possible.