Pharmacological properties of the drug Dostinex
A dopaminergic ergoline derivative with a pronounced and long-lasting prolactin-lowering effect. The drug inhibits prolactin secretion by directly stimulating D2-dopamine receptors in lactotrophic pituitary cells. In addition, when taken in higher doses than those used to reduce prolactin secretion, cabergoline has a central dopaminergic effect due to stimulation of D2 receptors. A decrease in the level of prolactin in the blood plasma is observed 3 hours after taking the drug orally and persists for 7–28 days in healthy volunteers and patients with hyperprolactinemia and up to 14–21 days when taken to suppress postpartum lactation. Dostinex is rapidly absorbed from the digestive tract, the maximum concentration in the blood plasma is reached after 0.5–4 hours. Food intake does not affect the absorption and distribution of cabergoline. The half-life, estimated from the rate of urinary excretion, is 63–68 hours in healthy volunteers and 79–115 hours in patients with hyperprolactinemia. Due to the long half-life, steady state is achieved after 4 weeks. About 41–42% of the drug binds to blood plasma proteins.
Indications for use of the drug Dostinex
Prevention/suppression of physiological lactation. Prescribed to prevent physiological postpartum lactation or suppress established lactation. Treatment of hyperprolactinemia. Dostinex is also prescribed for the treatment of hyperprolactinemia, manifested by menstrual irregularities (amenorrhea, oligomenorrhea, anovulation), infertility, galactorrhea in women or impotence, decreased libido in men. Dostinex is indicated for the treatment of patients with prolactin-secreting pituitary adenomas (micro- and macroprolactinomas), idiopathic hyperprolactinemia or empty sella syndrome, which are the main pathological conditions associated with hyperprolactinemia.
Use of the drug Dostinex
To prevent postpartum lactation, 1 mg is prescribed once on the first day after birth; to suppress established lactation - 0.25 mg (1/2 tablet) every 12 hours for 2 days. Treatment of hyperprolactinemia: Dostinex is taken 1-2 times a week (for example, on Monday or Monday and Thursday). You should start taking it with lower doses - 0.25 mg (1/2 tablet) or 0.5 mg (1 tablet) per week and, if necessary, increase the dose depending on the therapeutic effect and tolerability. The weekly dose should be increased gradually - by 0.5 mg at monthly intervals. Typically the therapeutic dose is 1 mg/week and can range from 0.25 to 2 mg/week. Doses up to 4.5 mg/week have been used to treat patients with hyperprolactinemia. A dose of 1 mg per week or higher should be taken in 2 divided doses (or more often) depending on tolerance. When establishing the dose, it is necessary to examine the patient to determine the minimum effective therapeutic dose. After an effective dosage regimen has been selected, it is advisable to regularly (once a month) determine the level of prolactin in the blood serum. Normalization of prolactin levels is usually observed within 2–4 weeks of treatment.
Dostinex 0.5 mg tab No. 2
Before prescribing Dostinex® for the treatment of disorders associated with hyperprolactinemia, it is necessary to conduct a complete study of pituitary function.
In addition, the state of the cardiovascular system should be assessed, including echocardiography, in order to identify asymptomatic valvular dysfunctions.
As with other ergot derivatives, pleural effusion/pleural fibrosis and valvulopathy have been observed in patients following long-term use of cabergoline. In some cases, patients had received prior therapy with ergotonine dopamine agonists. Therefore, Dostinex® should not be used in patients with signs and/or clinical symptoms of impaired cardiac or respiratory function associated with fibrotic changes or with a history of such conditions. The drug should be discontinued if signs of the appearance or worsening of blood regurgitation, narrowing of the lumen of the valves or thickening of the valve leaflets are detected (see section “Contraindications”).
The erythrocyte sedimentation rate has been found to increase with the development of pleural effusion or fibrosis. If an unexplained increase in erythrocyte sedimentation rate is detected, a chest x-ray is recommended. A study of the concentration of creatinine in the blood plasma and an assessment of renal function can also help in making a diagnosis. After discontinuation of Dostinex®, patients with pleural effusion/pleural fibrosis or valvulopathy experienced improvement in symptoms.
It is unknown whether cabergoline can worsen the condition of patients with signs of blood regurgitation. Cabergoline should not be used if fibrous lesions of the heart valve apparatus are detected (see section “Contraindications”).
Fibrotic disorders can develop asymptomatically. In this regard, the condition of patients receiving long-term therapy with cabergoline should be regularly monitored and special attention should be paid to the following symptoms:
- pleuropulmonary disorders: such as shortness of breath, difficulty breathing, persistent cough or chest pain;
- renal failure or obstruction of the vessels of the ureters or abdominal organs, which may be accompanied by pain in the side or lumbar region and swelling of the lower extremities, any swelling or tenderness when touched in the abdomen, which may indicate the development of retroperitoneal fibrosis;
— pericardial fibrosis and fibrosis of the heart valve leaflets often manifest as heart failure. In this regard, it is necessary to exclude fibrosis of the heart valves (and constrictive pericarditis) when symptoms of heart failure appear.
The patient's condition should be regularly monitored for the development of fibrotic disorders. The first time EchoCG should be performed 3–6 months after the start of therapy. This study should then be carried out depending on the clinical assessment of the patient's condition, paying special attention to the symptoms described above, at least every 6-12 months of therapy.
The need for other monitoring methods (eg, physical examination, including cardiac auscultation, radiography, computed tomography) is assessed individually for each patient.
When increasing the dose, patients should be under medical supervision in order to establish the lowest effective dose that provides a therapeutic effect. Once an effective dosage regimen has been selected, it is recommended to regularly (once a month) determine the concentration of prolactin in the blood serum. Normalization of prolactin concentrations is usually observed within 2–4 weeks of treatment.
After discontinuation of Dostinex®, a relapse of hyperprolactinemia is usually observed, but some patients experience persistent suppression of prolactin concentrations for several months. In most women, ovulatory cycles persist for at least 6 months after discontinuation of Dostinex®.
Dostinex® restores ovulation and fertility in women with hyperprolactinemic hypogonadism. Since pregnancy may occur before menstruation returns, it is recommended that pregnancy tests be performed at least once every 4 weeks during the amenorrheic period, and after menstruation returns, whenever menstruation is more than 3 days late. Women who want to avoid pregnancy should use barrier methods of contraception during treatment with Dostinex®, as well as after discontinuation of the drug until anovulation recurs. Women who become pregnant should be under medical supervision to promptly identify symptoms of an enlarged pituitary gland, since during pregnancy it is possible that pre-existing pituitary tumors may increase in size.
Dostinex® should be prescribed in lower doses to patients with severe hepatic impairment (Child-Pugh class C) who are candidates for long-term drug therapy. When a single dose of 1 mg was administered to such patients, an increase in AUC (area under the concentration-time curve) was observed compared to healthy volunteers and patients with less severe hepatic impairment.
The use of cabergoline causes drowsiness. In patients with Parkinson's disease, the use of dopamine receptor agonists may cause sudden sleep onset. In such cases, it is recommended to reduce the dose of Dostinex® or discontinue therapy.
No studies have been conducted on the use of the drug in elderly patients with disorders associated with hyperprolactinemia. The safety and effectiveness of the drug in children under 16 years of age have not been established.
Impact on the ability to drive vehicles and machinery
Patients taking the drug Dostinex® should refrain from driving vehicles and machinery and other potentially dangerous activities that require concentration and speed of psychomotor reactions.
Side effects of the drug Dostinex
Dostinex is usually well tolerated. When used to prevent and suppress lactation, the most common symptoms observed are a decrease in blood pressure, dizziness, nausea, headache, insomnia, and abdominal pain (in most cases, these phenomena are mild and short-lived). When treating hyperprolactinemia, the most common symptoms are nausea, headache, decreased blood pressure, dizziness, abdominal pain, dyspepsia, gastritis, general weakness, constipation, breast tenderness, hot flushes, depression, paresthesia. Typically, these symptoms are moderate or mild, appear during the first 2 weeks of use and then go away on their own. When Dostinex is discontinued, unwanted reactions disappear within a few days. Side effects of the drug are dose-dependent. In case of severe or persistent side effects, a temporary dose reduction followed by a gradual increase (for example, by 0.25 mg/week for 2 weeks) is necessary.
Hormone replacement therapy for men with age-related androgen deficiency
Specialization: urology, andrology
Age-related androgen deficiency syndrome in men is a biochemical imbalance that occurs in adulthood due to insufficiency of androgens in the blood serum, often accompanied by a decrease in the body's sensitivity to androgens. As a rule, this leads to a significant deterioration in the quality of life and adversely affects the functions of almost all body systems. Naturally, the issues of androgen deficiency therapy are of great interest, since it poses a difficult task for the clinician: to choose from a wide arsenal of methods and drugs of hormonal therapy the most optimal one, combining quality, efficiency, and ease of use.
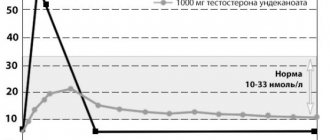
Currently, urologists and andrologists most often use testosterone replacement therapy. This method allows you to solve a number of problems: reduce the symptoms of age-related androgen deficiency by increasing libido and overall sexual satisfaction, reduce the severity or completely eliminate vegetative-vascular and mental disorders. In addition, if testosterone replacement therapy is used for more than 1 year, patients experience an increase in bone mass density, a decrease in the severity of visceral obesity, and an increase in muscle mass. Also, after a long course of treatment, laboratory parameters are normalized: there is an increase in hemoglobin levels or the number of red blood cells, a decrease in the level of VLDL (very low density lipoproteins) and LDL (low density lipoproteins) with an unchanged level of HDL (high density lipoproteins). Many authors believe that this effect can be achieved by restoring the concentration of testosterone in the blood to normal levels (10–35 nmol/l). It should also be taken into account that *17α-alkylated testosterone preparations fluoxymesterolone and methyltestosterone have pronounced hepatotoxicity, having a toxic and carcinogenic effect on the liver, and also negatively affect the lipid spectrum of the blood (a sharp increase in the level of atherogenic and a decrease in the level of antiatherogenic lipoproteins). Therefore, the use of these testosterone derivatives in clinical practice was discontinued. Currently, testosterone undecanoate (Andriol) is the preferred oral drug. This testosterone ester does not undergo primary hepatic metabolism, as it is absorbed into the lymphatic system, bypassing the liver. After hydrolysis of testosterone undecanoate in the lymphatic system, testosterone enters the systemic circulation, which has a therapeutic effect both on its own and through its main metabolites - dihydrotestosterone (DHT) and estradiol, which determine the full spectrum of androgenic activity of testosterone. Thus, testosterone undecanoate retains its activity when administered orally. At the same time, bypassing the portal vein system and passing through the liver, testosterone undecanoate does not have a hepatotoxic or hepatocarcinogenic effect. The half-life of the drug from plasma is 3–4 hours. In connection with this, the dosage regimen for testosterone undecanoate is 2 times a day, which is not always convenient for patients. Based on our own experience, we believe that Andriol is a fairly mild drug and helps only in cases of initial and minimal manifestations of age-related androgen deficiency.
Intramuscular injections of long-acting testosterone esters are also a widely used method of replacement therapy in men with hypogonadism. The two most well-known testosterone esters, testosterone cypionate and testosterone enanthate, have similar pharmacokinetics. When these drugs are administered intramuscularly, a depot is created from which the drug is released into the bloodstream. During the first 2–3 days after administration, testosterone levels rise to supraphysiological levels, and then slowly decrease over the next 2 weeks to subnormal values. The positive side of these drugs is the duration of the therapeutic effect. Nevertheless, sudden changes in testosterone levels, often felt by the patient himself in the form of increases and decreases in libido, general well-being, and emotional status, are undesirable qualities of these drugs. In this regard, great hopes are placed on the new drug Nebido (Schering), the pharmacokinetics of which are significantly different from other testosterone esters. Nebido is testosterone undecanoate and is a non-peak drug. Over the past two decades, much attention has been paid to research into the benefits of transdermal use of testosterone preparations. Scrotal patches are effective and some patients find them the most convenient treatment option. Skin patches are most well accepted by patients and provide effective serum testosterone levels. However, there are some differences between the two types of patches regarding their allergenic potential: skin patches have a much higher incidence of allergic reactions and skin irritation than scrotal patches. Testosterone gel has all the benefits of patches and does not cause skin reactions. Its only drawback is the possibility of contact of the gel with a partner and the insufficient number of long-term studies on its use. The transdermal route of testosterone administration avoids its primary metabolism in the liver and inactivation, as occurs with the use of oral androgen drugs, and also allows it to simulate the circadian rhythms of release of physiological unmodified testosterone and its natural metabolites, estradiol and DHT. In addition, therapy using patches and gel can be easily interrupted if necessary. The positive aspects of this treatment method also include the low risk of drug dependence. The European drug 5-α-dihydrotestosterone gel (DHT), although found to be effective, is unknown whether the isolated use of a non-aromatized androgen, such as DHT, has the same effect as testosterone, due to the fact that testosterone metabolites include estradiol. According to many authors, the use of the drug is not recommended, since DHT, due to the inability to convert into estradiol, does not have the full range of therapeutic properties of testosterone (for example, effects on bone tissue and the cardiovascular system). Some of the replacement therapy drugs, such as testosterone undecanoate, DHT gel and scrotal patches, cause a significant increase in serum DHT concentrations. DHT is known as the main androgen of the prostate, and there has been much discussion regarding its ability to cause prostate disease. However, despite these assumptions, in the last 10 years there has been no evidence of an increase in the incidence of prostate pathology when prescribing DHT drugs.
Thus, there are many drugs for androgen replacement therapy, but they all have certain side effects and also have an inhibitory effect on spermatogenesis. Recently, more and more studies have appeared demonstrating the secondary nature of age-related androgen deficiency. According to WHO materials, data were obtained on the preservation of the secreting function of Leydig cells in elderly men, which allowed scientists to propose a fundamentally new approach to the treatment of age-related androgen deficiency, based on stimulation of the synthesis of endogenous testosterone. However, we should not forget that along with absolute contraindications for androgen replacement therapy (breast and prostate cancer), there are also additional ones (benign prostatic hyperplasia with severe obstruction, prolactinoma, polycythemia). Relative contraindications include sleep disorders in the form of apnea, obstructive pulmonary diseases, and heavy smoking. Side effects of androgens include increased sleep apnea, polycythemia, gynecomastia, priapism, fluid retention, increased blood pressure, edema, increased prostate size, and inhibition of spermatogenesis. That is, today there are methods for treating age-related androgen deficiency, which can be divided into two groups, fundamentally different in their mechanism of action: replacement therapy with exogenous androgen drugs; therapy that stimulates the synthesis of endogenous testosterone. Thus, we can say that there is no optimal treatment for age-related androgen deficiency in men. And the choice of drug should be approached strictly individually, taking into account the patient’s age, body mass index, the need to preserve spermatogenesis, hematocrit values and concomitant diseases.
Bibliography:
- Kalinchenko S. Yu. Age-related androgen deficiency in men // Medical newspaper. – June 28, 2002 – No. 49.
- Cunningham GR Management of male aging: which testosterone replacement therapy should be used? The Aging Male 2000;3:203-209.
- Jordan WP Allergy and topical irritation associated with transdermal testosteron administration: a comparison of scrotal and nonscrotal transdermal systems. Am J Contact Dermat 1997;8:108-13.
- Yu Z, Gupta SK, Hwang SS, et al. Testosterone pharmakokinetics after application of an investigational transdermal system in hypogonadal men. J Clin Pharmacol 1997;37:1139-45.
- Gorpinchenko I. I., Miroshnikov Ya. O. Erectile dysfunction. – Lviv: Medicine of the World, 2003. – 80 p.
- Duncan C. Gould Hypoandrogen-metabolic (HAM) syndrome: an important men's health issue//IMNG.– 2007. – V.2. – P. 174–178.
- Shabsigh R. Testosterone therapy in erectile dysfunction and hypogonadism//J. Sex. Med. – 2005. – V.2. – P. 785–792.
- Traish AM, Kim N. Weapons of penile smooth muscle destruction: androgen deficiency promotes accumulation of adiposities in the corpus cavernosum // Aging male. – 2005. – V.8. – P. 141–146.
The material was published in the specialized publication for doctors ProTest, issue 5, May 2021. When using materials, a link to the journal is required.
Special instructions for the use of Dostinex
Are common. As with other ergot alkaloids, Dostinex is used with caution in patients with severe cardiovascular disease, Raynaud's syndrome, stomach ulcers or gastrointestinal bleeding, or with a history of severe mental illness. Liver failure. In patients with severe liver failure who use Dostinex for a long time, the lowest doses should be used. In patients with severe hepatic insufficiency (Child-Pugh class C) who used a single dose of 1 mg, an increase in AUC was observed in contrast to healthy individuals and patients with mild forms of hepatic insufficiency. Postural hypotension. During the period of Dostinex use, patients experienced postural hypotension. Therefore, it should be used with caution with drugs that lower blood pressure. Fibrosis/valvulopathy. Cases of pleural effusion/pulmonary fibrosis and valvulopathy have been reported in patients using ergot derivatives, including long-term use of cabergoline. Some cases involved patients who had previously used dopamine agonists. Therefore, it is not recommended to use the drug in patients who currently have clinical symptoms (or a history of them) of respiratory and cardiovascular diseases associated with tissue fibrosis. It is known that in patients who used Dostinex, pleural exudate or fibrosis may appear against the background of an increase in ESR. If there is an unmotivated increase in ESR, patients are recommended to undergo an X-ray examination of the lungs. Determination of serum creatinine levels can also be used as an auxiliary method in the diagnosis of fibrotic diseases. Discontinuation of cabergoline in the event of pleural effusion, pulmonary fibrosis or valvulopathy leads to regression of clinical symptoms. Before starting cabergoline treatment, all patients are recommended to undergo a cardiac examination, including an echocardiogram, to assess the presence of latent forms of heart valve pathology. In the future, it is recommended to regularly conduct clinical diagnostics to monitor the development of valve disease or fibrosis (physical examination, radiography, echocardiography, CT scan). Sleep disturbance/sudden falling asleep. The use of cabergoline causes drowsiness. Dopamine agonists may cause sudden sleep onset in patients with Parkinson's disease. If such cases occur, it is necessary to reduce the dose or discontinue treatment. Prevention/suppression of physiological lactation. By analogy with other horn drugs, Dostinex can be used by patients with hypertension, pregnancy-induced preeclampsia or postpartum hypertension, only in cases where the benefits of using the drug outweigh the possible risks. A single dose of 0.25 mg of Dostinex should not be exceeded in women during postpartum lactation (if it is suppressed) to prevent postural hypertension. Treatment of hyperprolactinemia. Before starting treatment for hyperprolactinemia with Dostinex, it is necessary to diagnose the pituitary gland. The drug restores ovulation and fertility in women with hyperprolactinemic hypogonadism, therefore it is recommended to carry out a pregnancy test every 4 weeks during the amenorrhea period and after each restoration of menstruation if their delay is more than 3 days. Women are recommended to use mechanical contraception during Dostinex therapy and after its discontinuation until anovulation returns. Women who have become pregnant should be constantly monitored for symptoms of pituitary enlargement, since the clinical manifestations of a pituitary tumor may return. Mental disorders. In patients who use dopamine receptor antagonists, including Dostinex, cases of the development of a pathological tendency to gambling, increased libido and hypersexuality have been recorded. These effects are usually reversible after discontinuation of therapy. The ability to influence the reaction rate when driving a vehicle or working with other mechanisms. Patients using cabergoline who are prone to drowsiness should be advised to refrain from driving or performing activities that require alertness, except in patients who are able to overcome episodes of drowsiness, due to the risk of serious injury. or even the death of the patient or others.
Dostinex®
Before prescribing Dostinex for the treatment of disorders associated with hyperprolactinemia, it is necessary to conduct a complete study of pituitary function.
When increasing the dose, patients should be under medical supervision in order to establish the lowest effective dose that provides a therapeutic effect. Once an effective dosage regimen has been selected, it is recommended to regularly (once a month) determine the concentration of prolactin in the blood serum. Normalization of prolactin levels is usually observed within 2-4 weeks of treatment.
After discontinuation of Dostinex, a relapse of hyperprolactinemia is usually observed, but some patients experience persistent suppression of prolactin levels for several months. In most women, ovulatory cycles persist for at least 6 months after discontinuation of Dostinex.
Dostinex® restores ovulation and fertility in women with hyperprolactinemic hypogonadism. Since pregnancy may occur before menstruation returns, it is recommended that pregnancy tests be performed at least once every 4 weeks during the amenorrheic period, and after menstruation returns, whenever menstruation is more than 3 days late. Women who want to avoid pregnancy should use barrier methods of contraception during treatment with Dostinex, as well as after discontinuation of the drug until anovulation recurs. Women who become pregnant should be under medical supervision to promptly identify symptoms of an enlarged pituitary gland, since pre-existing pituitary tumors may increase in size during pregnancy.
Dostinex® should be prescribed in lower doses to patients with severe hepatic impairment (Child-Pugh class C) who are candidates for long-term drug therapy. With a single dose of 1 mg administered to such patients, an increase in AUC was observed compared with healthy volunteers and patients with less severe hepatic impairment.
As with other ergot derivatives, pleural effusion/pleural fibrosis and valvulopathy have been observed in patients following long-term use of cabergoline. In some cases, patients had received prior therapy with ergotinine dopamine agonists. Therefore, Dostinex® should be used with caution in patients with existing signs and/or clinical symptoms of cardiac dysfunction or with a history of such conditions. After discontinuation of Dostinex, patients diagnosed with pleural effusion/pleural fibrosis and valvulopathy experienced improvement in symptoms.
The use of cabergoline causes drowsiness. In patients with Parkinson's disease, the use of dopamine receptor agonists may cause sudden sleep onset. In such cases, it is recommended to reduce the dose of Dostinex or discontinue therapy.
No studies have been conducted on the use of the drug in elderly patients with disorders associated with hyperprolactinemia.
Use in pediatrics
Safety and effectiveness of the drug in children under 16 years of age
not installed.
Impact on the ability to drive vehicles and operate machinery
Patients taking Dostinex® who experience drowsiness should be warned that they should refrain from driving a vehicle or performing work (for example, operating machinery) in which decreased alertness could put them or others at risk of serious injury or of death.
Interactions of the drug Dostinex
No interaction was observed with concomitant therapy with methylergonovine maleate in early pregnancy. Despite the fact that there is no data on the interaction of Dostinex with other ergot alkaloids, the simultaneous administration of these drugs for a long time is not recommended. Since the effect of Dostinex is realized through direct stimulation of dopamine receptors, simultaneous administration of dopamine antagonists (for example, phenothiazines, butyrophenones, thioxanthenes, metoclopramide) is not recommended, since this may reduce the clinical effectiveness of Dostinex. When taking Dostinex, symptomatic arterial hypotension may develop, so Dostinex should be prescribed with caution simultaneously with other drugs that lower blood pressure.