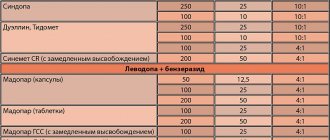
Levodopa/Benserazid-Teva 100mg+25mg tab No. 100
Dosage
100 mg+25 mg
Active substance
Levodopa + Benserazide
Manufacturer
Teva Pharmaceutical Works Private Limited Company (Hungary)
Shelf life
2 years
Storage conditions
In a place protected from moisture, at a temperature not exceeding 25 °C
Registration certificate number
LP-001827 dated 01/31/2019
Compound
| Pills | 1 table |
| active substances: | |
| levodopa | 100/200 mg |
| benserazide | 25/50 mg |
| corresponds to benserazide hydrochloride - 28.5/57 mg | |
| excipients: mannitol - 89.15/178.3 mg; MCC - 4.95/9.9 mg; pregelatinized corn starch - 18.7/37.4 mg; calcium hydrogen phosphate (anhydrous) - 7.97/15.94 mg; povidone - K25 11/22 mg; crospovidone (type A) - 8.25/16.5 mg; colloidal silicon dioxide - 0.71/1.42 mg; red iron oxide dye (E172) - 0.27/0.54 mg; magnesium stearate – 5.5/11 mg |
Characteristic
| Pills | 1 table |
| active substances: | |
| levodopa | 100/200 mg |
| benserazide | 25/50 mg |
| corresponds to benserazide hydrochloride - 28.5/57 mg | |
| excipients: mannitol - 89.15/178.3 mg; MCC - 4.95/9.9 mg; pregelatinized corn starch - 18.7/37.4 mg; calcium hydrogen phosphate (anhydrous) - 7.97/15.94 mg; povidone - K25 11/22 mg; crospovidone (type A) - 8.25/16.5 mg; colloidal silicon dioxide - 0.71/1.42 mg; red iron oxide dye (E172) - 0.27/0.54 mg; magnesium stearate – 5.5/11 mg |
Description of the dosage form
Dosage 100 mg+25 mg:
round, biconvex tablets, pink in color with slight marbling, with a cross-shaped score on both sides.
Dosage 200 mg+50 mg:
Round, flat, beveled tablets, pink in color, with slight marbling. There is a cross-shaped mark on both sides of the tablet. On one side there is an engraving “B” and “L” in two sections of a cross-shaped mark.
Pharmacokinetics
Suction.
Levodopa and benserazide are absorbed primarily in the upper small intestine. Tmax when taken orally is approximately 1 hour. AUC and Cmax are proportional to the dose taken. Absorption depends on the rate of gastric emptying and intragastric pH values. The presence of food in the stomach slows down absorption. When using levodopa after a normal meal, the Cmax of levodopa in plasma is 30% less and is achieved later. The degree of absorption is reduced by 15%. It is found in large quantities in the small intestine, liver and kidneys, only about 1–3% reaches the brain. T1/2 - 3 hours.
Distribution.
Levodopa crosses the BBB via a saturable transport system. It does not bind to plasma proteins. Vd - 57 l. The AUC of levodopa in cerebrospinal fluid is 12% of that in plasma.
Unlike levodopa, benserazide does not penetrate the BBB. It accumulates mainly in the kidneys, lungs, small intestine and liver and penetrates the placental barrier.
Metabolism.
Levodopa is metabolized primarily by two major pathways (decarboxylation and o-methylation) and two additional pathways (transamination and oxidation). Aromatic L-amino acid decarboxylase converts levodopa to dopamine. The main end products of this metabolic pathway are homovanillic and dihydroxyphenylacetic acids. Catechol-o-methyl-transferase methylates levodopa to form 3-o-methyldopa. T1/2 of this main metabolite is 15 hours, and in patients who have received therapeutic doses of the drug, its accumulation occurs. Decreased peripheral decarboxylation of levodopa when administered concomitantly with benserazide results in higher plasma concentrations of levodopa and 3-o-methyldopa and lower concentrations of catecholamines (dopamine, norepinephrine) and phenolcarboxylic acids (homovanillic acid, dihydrophenylacetic acid). In the intestinal mucosa and liver, benserazide is hydroxylated to form trihydroxybenzylhydrazine. This metabolite is a potent inhibitor of aromatic L-amino acid decarboxylase.
Excretion.
Against the background of peripheral inhibition of aromatic L-amino acid decarboxylase, T1/2 of levodopa is 1.5 hours. Levodopa clearance from plasma is 430 ml/min. Benserazide is almost completely eliminated by metabolism. Metabolites are excreted mainly by the kidneys (64%) and to a lesser extent by the intestines (24%).
Cumulation.
The absolute accumulation of levodopa in combination with benserazide averages 98% (from 74 to 112%).
Pharmacokinetics in special groups of patients
Kidney failure.
Less than 10% of unchanged levodopa/benserazide is excreted by the kidneys, so no dosage adjustment is required in patients with mild to moderate renal impairment.
In elderly patients (65–78 years) with Parkinson's disease.
T1/2 and AUC of levodopa increase by 25%, which is not a clinically significant change.
Pharmacodynamics
Levodopa/benserazide is a combination antiparkinsonian drug containing a dopamine precursor and an inhibitor of peripheral aromatic L-amino acid decarboxylase. In Parkinsonism, the neurotransmitter dopamine is produced in the basal ganglia in insufficient quantities. Replacement therapy is carried out by using levodopa, a direct metabolic precursor of dopamine. Most of levodopa is converted to dopamine in peripheral tissues (intestines, liver, kidneys, heart, stomach), which is not involved in the antiparkinsonian effect, since peripheral dopamine penetrates the BBB poorly and is also responsible for most of its adverse reactions. Blocking extracerebral decarboxylation of levodopa is highly desirable. This is achieved by the simultaneous administration of levodopa and benserazide, an inhibitor of peripheral aromatic L-amino acid decarboxylase, which reduces the formation of dopamine in peripheral tissues, which indirectly leads to an increase in the amount of levodopa entering the central nervous system, on the one hand, and to a decrease in the manifestations of undesirable reactions of levodopa, on the other. another. A 4:1 combination of these substances is as effective as high-dose levodopa.
Contraindications
hypersensitivity to levodopa, benserazide or any other component of the drug;
severe dysfunction of the endocrine system;
glaucoma;
severe liver dysfunction;
severe renal impairment;
severe dysfunction of the cardiovascular system;
endogenous and exogenous psychoses;
simultaneous use with non-selective MAO inhibitors, a combination of MAO type A and MAO type B inhibitors (which is equivalent to non-selective MAO inhibition);
women of childbearing age who do not use reliable methods of contraception;
pregnancy;
breastfeeding period;
age up to 25 years.
Use during pregnancy and breastfeeding
Levodopa/Benserazide-Teva is contraindicated during pregnancy and in women of childbearing age who do not use reliable methods of contraception. If pregnancy is suspected, the drug should be discontinued immediately.
If it is necessary to take the drug, breastfeeding should be stopped, since skeletal development disorders in the child cannot be ruled out.
Directions for use and doses
Inside
, if possible, at least 30 minutes before or 1 hour after meals.
Treatment begins with a small dose, gradually increasing the dose for each patient individually until a therapeutic effect is achieved. High doses should be avoided when taking the drug simultaneously.
The following dosage regimen instructions should be considered as general recommendations.
For patients who have not previously taken levodopa, an initial dose of 50 mg levodopa/12.5 mg benserazide 2–4 times daily (from 100–200 mg levodopa/25–50 mg benserazide per day) is prescribed. If well tolerated, the dose is increased by 50–100 mg levodopa/12.5–25 mg benserazide every 3 days until a therapeutic effect is achieved.
Further (after the initial) dose selection is carried out once a month. Typically, a therapeutic effect is observed when taking 200–400 mg of levodopa/50–100 mg of benserazide per day.
The maximum daily dose is 800 mg levodopa/200 mg benserazide.
The daily dose should be divided into 4 or more doses. The frequency of doses should be distributed to ensure optimal therapeutic effect.
If adverse reactions occur, it is necessary to either stop increasing the dose or reduce the daily dose.
The optimal therapeutic effect is usually achieved when taking 300–800 mg levodopa/100–200 mg benserazide.
For patients who have previously taken levodopa,
Levodopa/Benserazide-Teva should be started 12 hours after stopping levodopa.
The dose of the drug should be approximately 20% of the previous dose of levodopa in order to maintain the already achieved therapeutic effect. If necessary, the dose is increased according to the scheme described for patients who have not previously taken levodopa.
In patients who have previously taken levodopa in combination with an aromatic L-amino acid decarboxylase inhibitor,
Levodopa/Benserazide-Teva should be started 12 hours after stopping levodopa in combination with an aromatic L-amino acid decarboxylase inhibitor. To minimize the decrease in the already achieved therapeutic effectiveness, it is necessary to stop the previous therapy at night and start taking the drug Levodopa/Benserazide-Teva the next morning. If necessary, the dose is increased according to the scheme described for patients who have not previously taken levodopa.
For patients who have previously taken other antiparkinsonian drugs,
taking the drug Levodopa/Benserazide-Teva is possible. As soon as the therapeutic effect of Levodopa/Benserazide-Teva becomes apparent, it is necessary to reconsider the treatment regimen and reduce or discontinue the alternative drug.
Dosage regimens in special cases
Patients who experience severe motor fluctuations
It is recommended to take the daily dose more than 4 times a day without changing the daily dose itself.
In old age
Dose increases should occur more slowly.
Experience of use in children and adolescents
limited.
For renal and liver failure
mild to moderate dose adjustment is not required.
When spontaneous movements such as chorea or athetosis occur
in the later stages of treatment it is necessary to reduce the dose.
With long-term use of the drug
The occurrence of freezing episodes, weakening of the effect towards the end of the dosage period and the on-off phenomenon can be eliminated or significantly reduced by reducing the dose or using a lower dose, but more often. Subsequently, the dose can be increased again to enhance the effect of treatment.
If adverse reactions occur from the cardiovascular system
it is necessary to reduce the dose.
Side effects
The incidence of adverse reactions is classified according to the following criteria: very often - at least 10%; often - not less than 1% and less than 10%; sometimes - not less than 0.1% and less than 1%; rarely - not less than 0.01% and less than 0.1%; very rarely - less than 0.01%, including isolated messages.
From the hematopoietic system:
very rarely - hemolytic anemia, transient leukopenia, thrombocytopenia.
From the nervous system:
often - headache, dizziness, convulsions, spontaneous movement disorders (such as chorea and athetosis), episodes of freezing, weakening of the effect towards the end of the dose period, on-off phenomenon, increased manifestations of restless legs syndrome; very rarely - severe drowsiness, episodes of sudden drowsiness.
Mental disorders:
rarely - agitation, anxiety, depressed mood, insomnia, delirium, aggression, depression, anorexia, moderate enthusiasm, pathological gambling, hypersexuality, increased libido; very rarely - hallucinations, temporary disorientation.
From the SSS side:
very rarely - arrhythmias, orthostatic hypotension (weakens after reducing the dose of the drug), increased blood pressure; frequency unknown - hot flashes.
From the digestive system:
very rarely - nausea, vomiting, diarrhea, isolated cases of loss or change in taste, dryness of the oral mucosa; frequency unknown - gastrointestinal bleeding.
For the skin and subcutaneous tissues:
rarely - itchy skin, rash.
From the laboratory parameters:
infrequently - a transient increase in the activity of liver transaminases, alkaline phosphatase, an increase in the concentration of bilirubin, an increase in urea and creatinine in the blood, a change in the color of urine to red, darkening when standing.
Other:
frequency unknown - febrile fever, excessive sweating.
Interaction
Pharmacokinetic interactions
With simultaneous use of trihexyphenidyl (m-anticholinergic), the rate, but not the extent, of absorption of levodopa decreases.
Ferrous sulfate reduces the Cmax and AUC of levodopa by 30–50%; these changes are in some cases clinically significant.
When used simultaneously with antacids, the degree of absorption of levodopa/benserazide is reduced by 32%.
Metoclopramide increases the rate of absorption of levodopa.
Pharmacodynamic interactions
Antipsychotics, opioids and antihypertensive drugs containing reserpine inhibit the effect of levodopa/benserazide. If necessary, use the lowest doses of these drugs.
When used concomitantly, pyridoxine may reduce the antiparkinsonian effect of levodopa/benserazide.
Levodopa/benserazide should not be used with non-selective MAO inhibitors. If it is necessary to use levodopa/benserazide in patients receiving irreversible non-selective MAO inhibitors, at least 2 weeks should pass from the moment of stopping the MAO inhibitor before starting the dose. Premature (within 2 weeks after discontinuation) use of levodopa/benserazide after a non-selective MAO inhibitor (eg tranylcypromine) can cause a hypertensive crisis.
Selective MAO type B inhibitors (including selegiline, rasagiline) and selective MAO type A inhibitors (moclobemide) can be used during treatment with levodopa/benserazide. In certain cases, selegiline may increase the effect of levodopa/benserazide without causing a dangerous interaction. It is recommended to adjust the dose of levodopa/benserazide depending on the individual needs of the patient in terms of therapeutic efficacy and tolerability.
The combination of selective MAO inhibitors type B and selective MAO inhibitors type A is equivalent to taking a non-selective MAO inhibitor, therefore this combination should not be used with levodopa/benserazide.
If it is necessary to use antihypertensive drugs during treatment with levodopa/benserazide, the possibility of developing orthostatic hypotension must be taken into account.
Levodopa/benserazide potentiates the effect of sympathomimetics (epinephrine, norepinephrine, isoproterenol, amphetamine), so this combination of drugs should not be used. If simultaneous use is still necessary, then the state of the cardiovascular system should be carefully monitored and, if necessary, reduce the dose of sympathomimetics.
It is possible to use levodopa/benserazide with other antiparkinsonian drugs (anticholinergic drugs, amantadine, dopamine receptor agonists), and not only the desired but also the undesirable effects may be enhanced. It may be necessary to reduce the dose of levodopa/benserazide or other drug. When levodopa/benserazide is used concomitantly with a catechol-O-methyltransferase inhibitor, a reduction in the dose of levodopa/benserazide may be necessary.
Since a patient receiving levodopa/benserazide may experience fluctuations in blood pressure and arrhythmias during halothane anesthesia, it is necessary to discontinue the drug 12–48 hours before surgery.
Protein-rich foods may reduce the therapeutic effect of levodopa/benserazide.
Levodopa/benserazide may affect laboratory results of catecholamines, creatinine, uric acid, glucose, alkaline phosphatase, and bilirubin. An increase in the concentration of urea and creatinine in the blood, a false negative reaction to glucose in the urine when using the glucose oxidase method, and a false positive result from the Coombs test can be detected.
special instructions
Adverse reactions from the gastrointestinal tract, possible at the initial stage of treatment, are largely eliminated if Levodopa/Benserazide-Teva is taken with a small amount of food or liquid, as well as a slower dose increase. The use of Levodopa/Benserazide-Teva for the treatment of iatrogenic extrapyramidal syndrome and Huntington's chorea is not recommended.
In patients with a history of gastrointestinal ulcers, seizures and osteomalacia, it is necessary to regularly monitor the relevant indicators. During treatment, indicators of liver function, kidney function, and blood count should be monitored. In patients with a history of coronary artery disease, myocardial infarction, or cardiac arrhythmias, it is necessary to regularly monitor the ECG.
Patients with a history of orthostatic hypotension should be under medical supervision, especially at the beginning of treatment.
In patients with diabetes mellitus, blood glucose concentrations should be monitored frequently and the dose of oral hypoglycemic agents should be adjusted.
Cases of sudden onset of sleep have been reported with the use of Levodopa/Benserazide-Teva. Patients should be informed about the possibility of sudden sleep onset.
When using the drug Levodopa/Benserazide-Teva, the risk of developing malignant melanoma increases, and therefore the use of the drug in patients with malignant melanoma, incl. history, not recommended. The use of Levodopa/Benserazide-Teva, especially in high doses, increases the risk of developing compulsive disorders.
Before general anesthesia, Levodopa/Benserazid-Teva should be taken for as long a period as possible. An exception is halothane anesthesia. Since the patient receiving the drug may experience fluctuations in blood pressure and arrhythmias during halothane anesthesia, the drug should be discontinued 12–24 hours before surgery. After surgery, treatment is resumed, gradually increasing the dose.
Levodopa/Benserazid-Teva should not be discontinued abruptly. Abrupt withdrawal of the drug can lead to withdrawal syndrome (fever, muscle rigidity, as well as possible mental changes and increased CPK activity in the blood serum) or akinetic crises, which can take a life-threatening form. If such symptoms occur, the patient should be under medical supervision (if necessary, hospitalized) and receive appropriate therapy, which may include repeated use of Levodopa/Benserazide-Teva.
Depression can be a clinical manifestation of the underlying disease (parkinsonism) and can also occur during treatment with Levodopa/Benserazide-Teva. Such patients should be under medical supervision for timely identification of psychiatric adverse reactions.
Some patients with Parkinson's disease have experienced the emergence of behavioral and cognitive disorders as a result of uncontrolled use of increasing doses of the drug, despite the doctor's recommendations and a significant increase in therapeutic doses.
Experience with the use of Levodopa/Benserazide-Teva in people under 25 years of age is limited.
Impact on the ability to drive vehicles and work with equipment.
Patients who experience excessive daytime sleepiness or sudden sleep episodes should avoid driving or operating machinery. If these symptoms occur during treatment with Levodopa/Benserazide-Teva, dose reduction or discontinuation of therapy should be considered.
Conditions for dispensing from pharmacies
On prescription.
NPS-RU-00013-DOK-PHARM-23072016
Pharmgroups
Combined antiparkinsonian drug (dopamine precursor + aromatic L-amino acid decarboxylase peripheral inhibitor) (Antiparkinsonian drugs in combinations)
Pharmaceutical actions
antiparkinsonian
Levodopa
The drug should be taken orally, if possible, at least 30 minutes before or 1 hour after meals.
Treatment begins with a small dose, gradually increasing the dose for each patient individually until a therapeutic effect is achieved. High doses should be avoided when taking the drug simultaneously. The following dosage regimen instructions should be considered as general recommendations.
For patients who have not previously taken levodopa, an initial dose of 50 mg levodopa / 12.5 mg benserazide 2-4 times a day is prescribed (from 100-200 mg levodopa / 25-50 mg benserazide per day). If well tolerated, the dose is increased by 50-100 mg levodopa/12.5-25 mg benserazide every 3 days until a therapeutic effect is achieved.
Further (after the initial) dose selection is carried out once a month. Typically, a therapeutic effect is observed when taking 200-400 mg of levodopa / 50-100 mg of benserazide per day.
The maximum daily dose is 800 mg levodopa/200 mg benserazide.
The daily dose should be divided into 4 or more doses. The frequency of doses should be distributed to ensure optimal therapeutic effect. If adverse reactions occur, it is necessary to either stop increasing the dose or reduce the daily dose.
The optimal therapeutic effect is achieved, as a rule, by taking 300-800 mg of levodopa / 100-200 mg of benserazide.
For patients who have previously taken levodopa, Levodopa/Benserazide-Teva should be started 12 hours after stopping levodopa. The dose of the drug should be approximately 20% of the previous dose of levodopa in order to maintain the already achieved therapeutic effect. If necessary, the dose is increased according to the scheme described for patients who have not previously taken levodopa.
For patients who have previously taken levodopa in combination with an aromatic L-amino acid decarboxylase inhibitor, Levodopa/Benserazide-Teva should be started 12 hours after discontinuation of levodopa in combination with an aromatic L-amino acid decarboxylase inhibitor. To minimize the decrease in the already achieved therapeutic effectiveness, it is necessary to stop the previous therapy at night and start taking the drug Levodopa/Benserazide-Teva the next morning. If necessary, the dose is increased according to the scheme described for patients who have not previously taken levodopa.
Patients who have previously taken other antiparkinsonian drugs can take Levodopa/Benserazide-Teva. As soon as the therapeutic effect of Levodopa/Benserazide-Teva becomes apparent, it is necessary to reconsider the treatment regimen and reduce or discontinue the alternative drug.
Dosage regimens in special cases
For patients who experience severe motor fluctuations, it is recommended to take the daily dose more than 4 times a day without changing the daily dose itself. In old age, dose increases should occur more slowly. Experience with children and adolescents is limited.
For mild to moderate renal and liver failure
no dose adjustment is required.
If spontaneous movements such as chorea or athetosis appear in the later stages of treatment, it is necessary to reduce the dose.
With long-term use of the drug, the appearance of episodes of “freezing”, weakening of the effect towards the end of the dose period and the “on-off” phenomenon can be eliminated or significantly reduced by reducing the dose or using the drug in a lower dose, but more often. Subsequently, the dose can be increased again to enhance the effect of treatment.
If adverse reactions from the cardiovascular system occur, it is necessary to reduce the dose.